Greening under high light or cold temperature affects the level of xanthophyll-cycle pigments, early light-inducible proteins, and light-harvesting polypeptides in wild-type barley and the chlorina f2 mutant
- PMID: 10318697
- PMCID: PMC59251
- DOI: 10.1104/pp.120.1.193
Greening under high light or cold temperature affects the level of xanthophyll-cycle pigments, early light-inducible proteins, and light-harvesting polypeptides in wild-type barley and the chlorina f2 mutant
Abstract
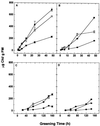
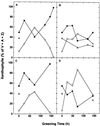
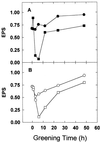
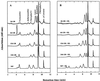
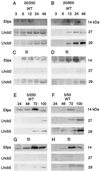
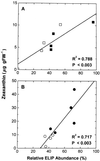
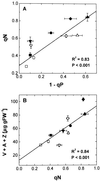
Etiolated seedlings of wild type and the chlorina f2 mutant of barley (Hordeum vulgare) were exposed to greening at either 5 degrees C or 20 degrees C and continuous illumination varying from 50 to 800 &mgr;mol m-2 s-1. Exposure to either moderate temperature and high light or low temperature and moderate light inhibited chlorophyll a and b accumulation in the wild type and in the f2 mutant. Continuous illumination under these greening conditions resulted in transient accumulations of zeaxanthin, concomitant transient decreases in violaxanthin, and fluctuations in the epoxidation state of the xanthophyll pool. Photoinhibition-induced xanthophyll-cycle activity was detectable after only 3 h of greening at 20 degrees C and 250 &mgr;mol m-2 s-1. Immunoblot analyses of the accumulation of the 14-kD early light-inducible protein but not the major (Lhcb2) or minor (Lhcb5) light-harvesting polypeptides demonstrated transient kinetics similar to those observed for zeaxanthin accumulation during greening at either 5 degrees C or 20 degrees C for both the wild type and the f2 mutant. Furthermore, greening of the f2 mutant at either 5 degrees C or 20 degrees C indicated that Lhcb2 is not essential for the regulation of the xanthophyll cycle in barley. These results are consistent with the thesis that early light-inducible proteins may bind zeaxanthin as well as other xanthophylls and dissipate excess light energy to protect the developing photosynthetic apparatus from excess excitation. We discuss the role of energy balance and photosystem II excitation pressure in the regulation of the xanthophyll cycle during chloroplast biogenesis in wild-type barley and the f2 mutant.
Figures

Similar articles
-
Barley's Second Spring as A Model Organism for Chloroplast Research.Plants (Basel). 2020 Jun 27;9(7):803. doi: 10.3390/plants9070803. Plants (Basel). 2020. PMID: 32604986 Free PMC article. Review.
-
Chlorophyll a/b-binding proteins, pigment conversions, and early light-induced proteins in a chlorophyll b-less barley mutant.Plant Physiol. 1995 Mar;107(3):873-83. doi: 10.1104/pp.107.3.873. Plant Physiol. 1995. PMID: 7748263 Free PMC article.
-
Regulation of the excitation energy utilization in the photosynthetic apparatus of chlorina f2 barley mutant grown under different irradiances.J Photochem Photobiol B. 2004 Jul 19;75(1-2):41-50. doi: 10.1016/j.jphotobiol.2004.04.004. J Photochem Photobiol B. 2004. PMID: 15246349
-
Photosystem II chlorophyll a fluorescence lifetimes and intensity are independent of the antenna size differences between barley wild-type and chlorina mutants: Photochemical quenching and xanthophyll cycle-dependent nonphotochemical quenching of fluorescence.Photosynth Res. 1996 May;48(1-2):171-87. doi: 10.1007/BF00041007. Photosynth Res. 1996. PMID: 24271297
-
Thermostability and Photostability of Photosystem II in Leaves of the Chlorina-f2 Barley Mutant Deficient in Light-Harvesting Chlorophyll a/b Protein Complexes.Plant Physiol. 1997 Mar;113(3):913-923. doi: 10.1104/pp.113.3.913. Plant Physiol. 1997. PMID: 12223653 Free PMC article.
Cited by
-
Preferential damaging effects of limited magnesium bioavailability on photosystem I in Sulla carnosa plants.Planta. 2015 May;241(5):1189-206. doi: 10.1007/s00425-015-2248-x. Epub 2015 Jan 31. Planta. 2015. PMID: 25637102
-
Barley's Second Spring as A Model Organism for Chloroplast Research.Plants (Basel). 2020 Jun 27;9(7):803. doi: 10.3390/plants9070803. Plants (Basel). 2020. PMID: 32604986 Free PMC article. Review.
-
Protein assembly and heat stability in developing thylakoid membranes during greening.Proc Natl Acad Sci U S A. 2002 Sep 17;99(19):12149-54. doi: 10.1073/pnas.192463899. Epub 2002 Sep 4. Proc Natl Acad Sci U S A. 2002. PMID: 12213965 Free PMC article.
-
Improvement of the Quality of Wild Rocket (Diplotaxis tenuifolia) with Respect to Health-Related Compounds by Enhanced Growth Irradiance.J Agric Food Chem. 2024 May 1;72(17):9735-9745. doi: 10.1021/acs.jafc.3c07698. Epub 2024 Apr 22. J Agric Food Chem. 2024. PMID: 38648561 Free PMC article.
-
Different Responses to Water Deficit of Two Common Winter Wheat Varieties: Physiological and Biochemical Characteristics.Plants (Basel). 2023 Jun 7;12(12):2239. doi: 10.3390/plants12122239. Plants (Basel). 2023. PMID: 37375865 Free PMC article.
References
-
- Adamska I. ELIPs: light induced stress proteins. Physiol Plant. 1997;100:794–805.
-
- Akoyunoglou G (1984) Thylakoid biogenesis in higher plants: assembly and reorganization. In C Sybesma, ed, Advances in Photosynthesis Research, Vol 4. Martinus Nijhoff/Dr W Junk Publishers, The Hague, The Netherlands, pp 595–602
-
- Baker NR. Development of chloroplast photochemical functions. In: Baker NR, Barber J, editors. Topics in Photosynthesis: Chloroplast Biogenesis, Vol 5. Amsterdam: Elsevier; 1984. pp. 207–251.
-
- Bassi R, Pineau B, Dainese P, Marquardt J. Carotenoid-binding proteins of photosystem II. Eur J Biochem. 1993;212:297–303. - PubMed
-
- Demmig-Adams B, Adams WW. Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:599–626.
LinkOut - more resources
Full Text Sources
Miscellaneous

