Identification of the soluble starch synthase activities of maize endosperm
- PMID: 10318698
- PMCID: PMC59252
- DOI: 10.1104/pp.120.1.205
Identification of the soluble starch synthase activities of maize endosperm
Abstract
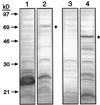
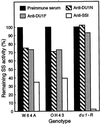
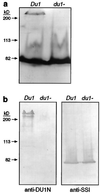
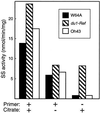
This study identified the complement of soluble starch synthases (SSs) present in developing maize (Zea mays) endosperm. The product of the du1 gene, DU1, was shown to be one of the two major soluble SSs. The C-terminal 450 residues of DU1 comprise eight sequence blocks conserved in 28 known or predicted glucan synthases. This region of DU1 was expressed in Escherichia coli and shown to possess SS activity. DU1-specific antisera detected a soluble endosperm protein of more than 200 kD that was lacking in du1- mutants. These antisera eliminated 20% to 30% of the soluble SS activity from kernel extracts. Antiserum against the isozyme zSSI eliminated approximately 60% of the total soluble SS, and immunodepletion of du1- mutant extracts with this antiserum nearly eliminated SS activity. Two soluble SS activities were identified by electrophoretic fractionation, each of which correlated specifically with zSSI or DU1. Thus, DU1 and zSSI accounted for the great majority of soluble SS activity present in developing endosperm. The relative activity of the two isozymes did not change significantly during the starch biosynthetic period. DU1 and zSSI may be interdependent, because mutant extracts lacking DU1 exhibited a significant stimulation of the remaining SS activity.
Figures

References
-
- Abel GJW, Springer F, Willmitzer L, Kossmann J. Cloning and functional analysis of a cDNA encoding a novel 139 kDa starch synthase from potato (Solanum tuberosum L.) Plant J. 1996;10:981–991. - PubMed
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Smith JA, Seidman JG, Struhl K. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1989.
-
- Ball S, Guan H-P, James M, Myers A, Keeling P, Mouille G, Buléon A, Colonna P, Preiss J. From glycogen to amylopectin: a model for the biogenesis of the plant starch granule. Cell. 1996;86:349–352. - PubMed
-
- Ball SG, van de Wal MHBJ, Visser RGF. Recent progress in understanding the biosynthesis of amylose. Trends Plant Sci. 1998;3:462–467.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

