Bivalency as a principle for proteasome inhibition
- PMID: 10318898
- PMCID: PMC21874
- DOI: 10.1073/pnas.96.10.5418
Bivalency as a principle for proteasome inhibition
Abstract
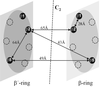
The proteasome, a multicatalytic protease, is known to degrade unfolded polypeptides with low specificity in substrate selection and cleavage pattern. This lack of well-defined substrate specificities makes the design of peptide-based highly selective inhibitors extremely difficult. However, the x-ray structure of the proteasome from Saccharomyces cerevisiae reveals a unique topography of the six active sites in the inner chamber of the protease, which lends itself to strategies of specific multivalent inhibition. Structure-derived active site separation distances were exploited for the design of homo- and heterobivalent inhibitors based on peptide aldehyde head groups and polyoxyethylene as spacer element. Polyoxyethylene was chosen as a flexible, linear, and proteasome-resistant polymer to mimic unfolded polypeptide chains and thus to allow access to the proteolytic chamber. Spacer lengths were selected that satisfy the inter- and intra-ring distances for occupation of the active sites from the S subsites. X-ray analysis of the proteasome/bivalent inhibitor complexes confirmed independent recognition and binding of the inhibitory head groups. Their inhibitory potencies, which are by 2 orders of magnitude enhanced, compared with pegylated monovalent inhibitors, result from the bivalent binding. The principle of multivalency, ubiquitous in nature, has been successfully applied in the past to enhance affinity and avidity of ligands in molecular recognition processes. The present study confirms its utility also for inhibition of multicatalytic protease complexes.
Figures




References
-
- Groettrup M, Schmidtke G. DDT. 1999;4:63–71. - PubMed
-
- Goldberg A L, Stein R, Adams J. Chem Biol. 1995;2:503–508. - PubMed
-
- Hochstrasser M. Curr Opin Cell Biol. 1995;7:215–223. - PubMed
-
- Coux O, Tanaka K, Goldberg A L. Annu Rev Biochem. 1996;65:801–847. - PubMed
-
- Stock D, Nederlof P M, Seemüller E, Baumeister W, Huber R, Löwe J. Curr Opin Biotechnol. 1996;7:376–385. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

