From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells
- PMID: 10318905
- PMCID: PMC21881
- DOI: 10.1073/pnas.96.10.5458
From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells
Abstract
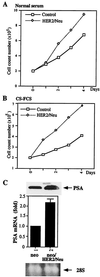
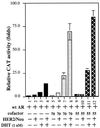
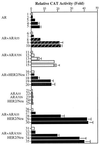
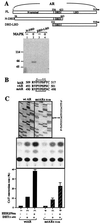
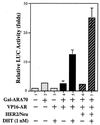
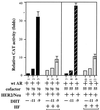
Overexpression of the HER2/Neu protooncogene has been linked to the progression of breast cancer. Here we demonstrate that the growth of prostate cancer LNCaP cells can also be increased by the stable transfection of HER2/Neu. Using AG879, a HER2/Neu inhibitor, and PD98059, a MAP kinase inhibitor, as well as MAP kinase phosphatase-1 (MPK-1), in the transfection assay, we found that HER2/Neu could induce prostate-specific antigen (PSA), a marker for the progression of prostate cancer, through the MAP kinase pathway at a low androgen level. Reporter assays and mammalian two-hybrid assays further suggest this HER2/Neu-induced androgen receptor (AR) transactivation may function through the promotion of interaction between AR and AR coactivators, such as ARA70. Furthermore, we found this HER2/Neu --> MAP kinase --> AR-ARAs --> PSA pathway could not be blocked completely by hydroxyflutamide, an antiandrogen used in the treatment of prostate cancer. Together, these data provide a novel pathway from HER2/Neu to AR transactivation, and they may represent one of the reasons for the PSA re-elevation and hormone resistance during androgen ablation therapy in prostate cancer patients.
Figures

References
-
- Harris A L, Nicholson S, Sainsbury R, Wright C, Farndon J. J Natl Cancer Inst Monogr. 1992;11:181–187. - PubMed
-
- Kwong K Y, Hung M C. Mol Carcinog. 1998;23:62–68. - PubMed
-
- Gullick W J, Berger M S, Bennett P L, Rothbard J B, Waterfield M D. Int J Cancer. 1987;40:246–254. - PubMed
-
- Press M F, Cordon-Cardo C, Slamon D J. Oncogene. 1990;5:953–962. - PubMed
-
- Muller W J, Sinn E, Pattengaale P K, Leader P. Cell. 1988;54:105–115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

