Bloom's syndrome protein, BLM, colocalizes with replication protein A in meiotic prophase nuclei of mammalian spermatocytes
- PMID: 10318934
- PMCID: PMC21910
- DOI: 10.1073/pnas.96.10.5622
Bloom's syndrome protein, BLM, colocalizes with replication protein A in meiotic prophase nuclei of mammalian spermatocytes
Abstract
Bloom's syndrome (BS) is a rare autosomal recessive disorder of humans characterized by severe pre- and postnatal growth deficiency, immunodeficiency, genomic instability, and a predisposition to a wide variety of neoplasms. The genomic instability is evidenced in BS somatic cells as a high incidence of gaps and breaks, chromatid exchanges, chromosome rearrangements, and locus-specific mutations. BS arises from a mutation in BLM, a gene encoding a protein with homology to the RecQ helicase family. Men with BS are sterile; women have reduced fertility and a shortened reproductive span. The current immunocytological study on mouse spermatocytes shows that the BLM protein is first evident as discrete foci along the synaptonemal complexes (SCs) of homologously synapsed autosomal bivalents in late zygonema of meiotic prophase. BLM foci progressively dissociate from the synapsed autosomal axes during early pachynema and are no longer seen in mid-pachynema. BLM colocalizes with the single-stranded DNA binding replication protein A, which has been shown to be involved in meiotic synapsis. However, there is a temporal delay in the appearance of BLM protein along the SCs relative to replication protein A, suggesting that BLM is required for a late step in processing of a subset of genomic DNA involved in establishment of interhomologue interactions in early meiotic prophase. In late pachynema and into diplonema, BLM is more dispersed in the nucleoplasm, especially over the chromatin most intimately associated with the SCs, suggesting a possible involvement of BLM in resolution of interlocks in preparation for homologous chromosome disjunction during anaphase I.
Figures
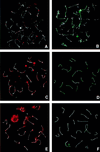
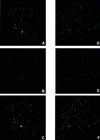
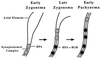
References
-
- German J, Ellis N A. In: The Genetic Basis of Human Cancer. Vogelstein B, Kenzler K W, editors. New York: McGraw–Hill; 1998. pp. 301–315.
-
- Ellis N A, Groden J, Ye T Z, Straughen J, Lennon D J, Ciocci S, Proytcheva M, German J. Cell. 1995;83:655–666. - PubMed
-
- Nakayama K, Irono N, Nakayama H. Mol Gen Genet. 1985;200:266–271. - PubMed
-
- Watt P M, Louis E J, Borts R H, Hickson I D. Cell. 1995;81:253–260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

