Defining therapeutic targets by using adenovirus: blocking NF-kappaB inhibits both inflammatory and destructive mechanisms in rheumatoid synovium but spares anti-inflammatory mediators
- PMID: 10318942
- PMCID: PMC21918
- DOI: 10.1073/pnas.96.10.5668
Defining therapeutic targets by using adenovirus: blocking NF-kappaB inhibits both inflammatory and destructive mechanisms in rheumatoid synovium but spares anti-inflammatory mediators
Abstract
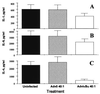
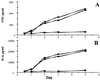
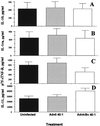
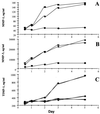
The role of the transcription factor NF-kappaB in the pathogenesis of rheumatoid arthritis has long been a subject of controversy. We used an adenoviral technique of blocking NF-kappaB through overexpression of the inhibitory subunit IkappaBalpha, which has the advantage that it can be used in the diseased tissue itself, with >90% of the synovial macrophages, fibroblasts, and T cells infected. We found that the spontaneous production of tumor necrosis factor alpha and other pro-inflammatory cytokines is NF-kappaB-dependent in rheumatoid synovial tissue, in contrast to the main anti-inflammatory mediators, like IL-10 and -11, and the IL-1 receptor antagonist. Of even more interest, IkappaBalpha overexpression inhibited the production of matrix metalloproteinases 1 and 3 while not affecting their tissue inhibitor. Blocking NF-kappaB in the rheumatoid joint thus has a very beneficial profile, reducing both the inflammatory response and the tissue destruction. The adenoviral technique described here has widespread applicability, allowing rapid testing of the effects of blocking a potential therapeutic target in either cultures of normal cells or in the diseased tissue itself.
Figures



References
-
- Elliott M J, Maini R N, Feldmann M, Long-Fox A, Charles P, Katsikis P, Brennan F M, Walker J, Bijl H, Ghrayeb J, et al. Arthritis Rheum. 1993;36:1681–1690. - PubMed
-
- Elliott M J, Maini R N, Feldmann M, Kalden J R, Antoni C, Smolen J S, Leeb B, Breedveld F C, Macfarlane J D, Bijl H, et al. Lancet. 1994;344:1105–1110. - PubMed
-
- Rankin E C C, Choy E H S, Kassimos D, Kingsley G H, Sopwith S M, Isenberg D A, Panayi G S. Br J Rheumatol. 1995;34:334–342. - PubMed
-
- Moreland L W, Baumgartner S W, Schiff M H, Tindall E A, Fleischmann R M, Weaver A L, Ettlinger R E, Cohen S, Koopman W J, Mohler K, et al. N Engl J Med. 1997;337:141–147. - PubMed
-
- Feldmann M, Elliott M J, Woody J N, Maini R N. Adv Immunol. 1997;64:283–350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

