Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers
- PMID: 10318946
- PMCID: PMC21922
- DOI: 10.1073/pnas.96.10.5692
Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers
Abstract
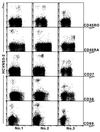
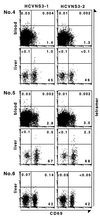
It is believed that the hepatitis C virus (HCV)-specific CD8(+) cytotoxic T lymphocytes (CTLs) play a role in the development of liver cell injury and in the clearance of the virus. To develop a direct binding assay for HCV-specific CTLs, we generated two peptide-MHC tetramers by using the recombinant HLA A2.1 molecule and A2-restricted T cell epitopes of the HCV NS3 protein. With these reagents we are able to detect specific CD8(+) cells in the blood of 15 of 20 HLA-A2(+), HCV-infected patients, at a frequency ranging from 0.01% to 1.2% of peripheral CD8(+) T cells. Phenotypic analysis of these specific cells indicated that there is a significant variation in the expression of the CD45 isoforms and CD27 in different patients. A 6-hour incubation of one patient's blood with NS3 peptides resulted in the activation of the epitope-specific CD8(+) cells, as indicated by their expression of CD69 and IFN-gamma. We also detected NS3-specific CD8(+) T cells in the intrahepatic lymphocyte population isolated from liver biopsies of two HCV-infected patients. The frequency of these specific CD8(+) cells in the liver was 1-2%, at least 30-fold higher than in the peripheral blood. All of the intrahepatic NS3-specific CD8(+) T cells were CD69(+), suggesting that they were activated CTLs. Direct quantitation and characterization of HCV-specific CTLs should extend our understanding of the immunopathogenesis and the mechanism of clearance or persistence of HCV.
Figures


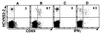
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

