A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity
- PMID: 10318947
- PMCID: PMC21923
- DOI: 10.1073/pnas.96.10.5698
A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity
Abstract
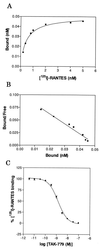
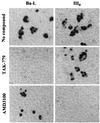
The beta-chemokine receptor CCR5 is considered to be an attractive target for inhibition of macrophage-tropic (CCR5-using or R5) HIV-1 replication because individuals having a nonfunctional receptor (a homozygous 32-bp deletion in the CCR5 coding region) are apparently normal but resistant to infection with R5 HIV-1. In this study, we found that TAK-779, a nonpeptide compound with a small molecular weight (Mr 531.13), antagonized the binding of RANTES (regulated on activation, normal T cell expressed and secreted) to CCR5-expressing Chinese hamster ovary cells and blocked CCR5-mediated Ca2+ signaling at nanomolar concentrations. The inhibition of beta-chemokine receptors by TAK-779 appeared to be specific to CCR5 because the compound antagonized CCR2b to a lesser extent but did not affect CCR1, CCR3, or CCR4. Consequently, TAK-779 displayed highly potent and selective inhibition of R5 HIV-1 replication without showing any cytotoxicity to the host cells. The compound inhibited the replication of R5 HIV-1 clinical isolates as well as a laboratory strain at a concentration of 1.6-3.7 nM in peripheral blood mononuclear cells, though it was totally inactive against T-cell line-tropic (CXCR4-using or X4) HIV-1.
Figures


References
-
- Havlir D V, Richman D D. Ann Intern Med. 1996;124:984–994. - PubMed
-
- Carpenter C C J, Fischl M A, Hammer S M, Hirsch M S, Jacobsen D M, Katzenstein D A, Montaner J S G, Richman D D, Saag M S, Schooley R T, et al. J Am Med Assoc. 1998;280:78–86. - PubMed
-
- Deeks S G, Smith M, Holodniy M, Kahn J O. J Am Med Assoc. 1997;277:145–153. - PubMed
-
- Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, et al. Nature (London) 1996;381:661–666. - PubMed
-
- Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, et al. Nature (London) 1996;381:667–673. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

