Trading force for speed: why superfast crossbridge kinetics leads to superlow forces
- PMID: 10318969
- PMCID: PMC21945
- DOI: 10.1073/pnas.96.10.5826
Trading force for speed: why superfast crossbridge kinetics leads to superlow forces
Abstract
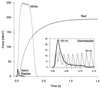
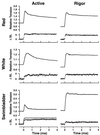
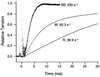
Superfast muscles power high-frequency motions such as sound production and visual tracking. As a class, these muscles also generate low forces. Using the toadfish swimbladder muscle, the fastest known vertebrate muscle, we examined the crossbridge kinetic rates responsible for high contraction rates and how these might affect force generation. Swimbladder fibers have evolved a 10-fold faster crossbridge detachment rate than fast-twitch locomotory fibers, but surprisingly the crossbridge attachment rate has remained unchanged. These kinetics result in very few crossbridges being attached during contraction of superfast fibers (only approximately 1/6 of that in locomotory fibers) and thus low force. This imbalance between attachment and detachment rates is likely to be a general mechanism that imposes a tradeoff of force for speed in all superfast fibers.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

