Genetic engineering of the unsaturation of fatty acids in membrane lipids alters the tolerance of Synechocystis to salt stress
- PMID: 10318975
- PMCID: PMC21951
- DOI: 10.1073/pnas.96.10.5862
Genetic engineering of the unsaturation of fatty acids in membrane lipids alters the tolerance of Synechocystis to salt stress
Abstract
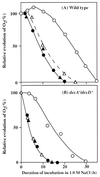
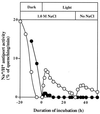
The role of unsaturated fatty acids in membrane lipids in the tolerance of the photosynthetic machinery to salt stress was studied by comparing the desA-/desD- mutant of Synechocystis sp. PCC 6803, which contained monounsaturated fatty acids, with the wild-type strain, which contained a full complement of polyunsaturated fatty acids. In darkness, the loss of oxygen-evolving photosystem II activity in the presence of 0.5 M NaCl or 0.5 M LiCl was much more rapid in desA-/desD- cells than in wild-type cells. Oxygen-evolving activity that had been lost during incubation with 0.5 M NaCl in darkness returned when cells were transferred to conditions that allowed photosynthesis or respiration. Recovery was much greater in wild-type than in desA-/desD- cells, and it was prevented by lincomycin. Thus, the unsaturation of fatty acids is important in the tolerance of the photosynthetic machinery to salt stress. It appears also that the activity and synthesis of the Na+/H+ antiporter system might be suppressed under high-salt conditions and that this effect can be reversed, in part, by the unsaturation of fatty acids in membrane lipids.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

