Diverse oxygenations catalyzed by carbazole 1,9a-dioxygenase from Pseudomonas sp. Strain CA10
- PMID: 10322011
- PMCID: PMC93765
- DOI: 10.1128/JB.181.10.3105-3113.1999
Diverse oxygenations catalyzed by carbazole 1,9a-dioxygenase from Pseudomonas sp. Strain CA10
Abstract
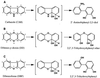
Carbazole 1,9a-dioxygenase (CARDO) from Pseudomonas sp. strain CA10 is a multicomponent enzyme that catalyzes the angular dioxygenation of carbazole, dibenzofuran, and dibenzo-p-dioxin. It was revealed by gas chromatography-mass spectrometry and 1H and 13C nuclear magnetic resonance analyses that xanthene and phenoxathiin were converted to 2,2',3-trihydroxydiphenylmethane and 2,2',3-trihydroxydiphenyl sulfide, respectively. Thus, for xanthene and phenoxathiin, angular dioxygenation by CARDO occurred at the angular position adjacent to the oxygen atom to yield hetero ring-cleaved compounds. In addition to the angular dioxygenation, CARDO catalyzed the cis dihydroxylation of polycyclic aromatic hydrocarbons and biphenyl. Naphthalene and biphenyl were converted by CARDO to cis-1, 2-dihydroxy-1,2-dihydronaphthalene and cis-2,3-dihydroxy-2, 3-dihydrobiphenyl, respectively. On the other hand, CARDO also catalyzed the monooxygenation of sulfur heteroatoms in dibenzothiophene and of the benzylic methylenic group in fluorene to yield dibenzothiophene-5-oxide and 9-hydroxyfluorene, respectively. These results indicate that CARDO has a broad substrate range and can catalyze diverse oxygenation: angular dioxygenation, cis dihydroxylation, and monooxygenation. The diverse oxygenation catalyzed by CARDO for several aromatic compounds might reflect the differences in the binding of the substrates to the reaction center of CARDO.
Figures


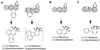
Similar articles
-
Identification and characterization of genes encoding carbazole 1,9a-dioxygenase in Pseudomonas sp. strain CA10.J Bacteriol. 1997 Aug;179(15):4850-8. doi: 10.1128/jb.179.15.4850-4858.1997. J Bacteriol. 1997. PMID: 9244274 Free PMC article.
-
Alteration of the substrate specificity of the angular dioxygenase carbazole 1,9a-dioxygenase.Biosci Biotechnol Biochem. 2008 Dec;72(12):3237-48. doi: 10.1271/bbb.80512. Epub 2008 Dec 7. Biosci Biotechnol Biochem. 2008. PMID: 19060398
-
Structure of the terminal oxygenase component of angular dioxygenase, carbazole 1,9a-dioxygenase.J Mol Biol. 2005 Aug 12;351(2):355-70. doi: 10.1016/j.jmb.2005.05.059. J Mol Biol. 2005. PMID: 16005887
-
Bacterial metabolism of fluorene, dibenzofuran, dibenzothiophene, and carbazole.Can J Microbiol. 2000 May;46(5):397-409. Can J Microbiol. 2000. PMID: 10872075 Review.
-
Structural and molecular genetic analyses of the bacterial carbazole degradation system.Biosci Biotechnol Biochem. 2012;76(1):1-18. doi: 10.1271/bbb.110620. Epub 2012 Jan 7. Biosci Biotechnol Biochem. 2012. PMID: 22232235 Review.
Cited by
-
Genomic and metabolic analysis of fluoranthene degradation pathway in Celeribacter indicus P73T.Sci Rep. 2015 Jan 13;5:7741. doi: 10.1038/srep07741. Sci Rep. 2015. PMID: 25582347 Free PMC article.
-
Genetic characterization and evolutionary implications of a car gene cluster in the carbazole degrader Pseudomonas sp. strain CA10.J Bacteriol. 2001 Jun;183(12):3663-79. doi: 10.1128/JB.183.12.3663-3679.2001. J Bacteriol. 2001. PMID: 11371531 Free PMC article.
-
Substrate-baited nanoparticles: a catch and release strategy for enzyme recognition and harvesting.Small. 2012 Jul 9;8(13):2083-90. doi: 10.1002/smll.201200013. Epub 2012 Apr 24. Small. 2012. PMID: 22532510 Free PMC article.
-
The genes coding for the conversion of carbazole to catechol are flanked by IS6100 elements in Sphingomonas sp. strain XLDN2-5.PLoS One. 2010 Apr 2;5(4):e10018. doi: 10.1371/journal.pone.0010018. PLoS One. 2010. PMID: 20368802 Free PMC article.
-
Simultaneous biodetoxification of S, N, and O pollutants by engineering of a carbazole-degrading gene cassette in a recombinant biocatalyst.Appl Environ Microbiol. 2006 Nov;72(11):7373-6. doi: 10.1128/AEM.01374-06. Epub 2006 Aug 25. Appl Environ Microbiol. 2006. PMID: 16936043 Free PMC article.
References
-
- Allen C C R, Boyd D R, Dalton H, Sharma N D, Haughey S A, McMordie R A S, McMurray B T, Sheldrake G N, Sproule K. Sulfoxides of high enantiopurity from bacterial oxygenase-catalyzed oxidation. J Chem Soc Chem Commun. 1995;1995:119–120.
-
- Cerniglia C E. Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation. 1992;3:351–368.
-
- Engesser K H, Strubel V, Christoglou K, Fischer P, Rast H G. Dioxygenolytic cleavage of aryl ether bonds: 1,10-dihydro-1,10-dihydroxyfluorene-9-one, a novel arene dihydrodiol as evidence for angular dioxygenation of dibenzofuran. FEMS Microbiol Lett. 1989;65:205–210. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

