Mechanisms underlying ACh induced modulation of neurogenic and applied ATP constrictions in the submucosal arterioles of the guinea-pig small intestine
- PMID: 10323595
- PMCID: PMC1565932
- DOI: 10.1038/sj.bjp.0702461
Mechanisms underlying ACh induced modulation of neurogenic and applied ATP constrictions in the submucosal arterioles of the guinea-pig small intestine
Abstract
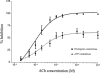
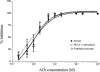
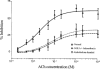
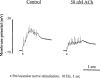
1. Role of the vascular endothelium in acetylcholine (ACh) induced modulation of neurogenic and applied ATP (adenosine 5'-triphosphate) constrictions of intestinal submucosal arterioles was investigated. 2. Arteriole constrictions, induced either by exogenous ATP or evoked by perivascular nerve stimulation, were attenuated in the presence of ACh. 100 nM ACh almost completely abolished neurogenic constrictions whereas up to 10 microM ACh reduced constrictions to exogenous ATP by only about 60%. 3. Treatment of the arterioles with 100 microM Nomega-nitro-L-arginine (NOLA) and 5 microM indomethacin, to block respectively nitric oxide (NO) and prostanoid release from the endothelium, had no effect on the ACh induced inhibition of neurogenic constrictions but significantly attenuated the inhibitory effects of ACh on constrictions to exogenous ATP. 4. Disruption of the vascular endothelium had no effect on the ACh induced inhibition of neurogenic constrictions but attenuated the inhibitory effects of ACh on applied ATP constrictions to the same extent as after treatment with NOLA and indomethacin. In comparison, endothelial disruption completely abolished the inhibitory effect of substance P (SP) on exogenously applied ATP constrictions. 5. 50 nM ACh significantly attenuated the amplitude of neurally evoked excitatory junction potentials (ejps) recorded from the vascular smooth muscle without altering the time constant of decay (taudecay) of the ejps. 6. It is concluded that ACh inhibits neurogenic constrictions by prejunctional modulation of transmitter release from the perivascular sympathetic nerves with no major role for endothelial paracrine factors. 7. Endothelial NO and/or prostanoids mediate some of the ACh induced inhibition of constrictions to exogenous ATP whereas the endothelium independent inhibitory effects of ACh are attributed to a direct action of ACh on the vascular smooth muscle. However, an indirect effect resulting from activation of vasodilator nerves cannot be ruled out.
Figures



Similar articles
-
Modulation of sympathetic nerve activity by perivascular sensory nerves in the arterioles of the guinea-pig small intestine.J Auton Nerv Syst. 1999 Sep 24;77(2-3):125-32. J Auton Nerv Syst. 1999. PMID: 10580294
-
Modulation of sympathetic nerve activity by perivascular sensory nerves in the arterioles of the guinea-pig small intestine.J Auton Nerv Syst. 1999 Sep 24;77(2-3):125-32. J Auton Nerv Syst. 1999. PMID: 11130956
-
NO mediates postjunctional inhibitory effect of neurogenic ACh in guinea pig small intestinal microcirculation.Am J Physiol. 1999 Oct;277(4):H1441-6. doi: 10.1152/ajpheart.1999.277.4.H1441. Am J Physiol. 1999. PMID: 10516180
-
TNF-alpha modulates arteriolar reactivity secondary to a change in intimal permeability.Microcirculation. 2000 Dec;7(6 Pt 1):411-8. Microcirculation. 2000. PMID: 11142338
-
Nitric oxide and the cerebral vascular function.J Biomed Sci. 2000 Jan-Feb;7(1):16-26. doi: 10.1007/BF02255914. J Biomed Sci. 2000. PMID: 10644885 Review.
Cited by
-
Purinergic signalling in the gastrointestinal tract and related organs in health and disease.Purinergic Signal. 2014 Mar;10(1):3-50. doi: 10.1007/s11302-013-9397-9. Epub 2013 Dec 4. Purinergic Signal. 2014. PMID: 24307520 Free PMC article. Review.
References
-
- ABBRACCHIO M.P., BURNSTOCK G. Purinoceptors: are there families of P2x and P2y purinoceptors. Pharmacol. Ther. 1994;64:445–475. - PubMed
-
- BEAN B.P. Pharmacology and electrophysiology of ATP-activated ion channels. Trends Pharmacol. Sci. 1992;13:87–90. - PubMed
-
- BENHAM C.D. ATP-gated ion channels in vascular smooth muscle. Jpn. J. Pharmacol. 1992;58 suppl 2:179P–184P. - PubMed
-
- BRAYDEN J.E., BEVAN J.A. Neurogenic muscarinic vasodilatation in the cat. An example of endothelial cell independent cholinergic relaxation. Circ. Res. 1985;56:205–211. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

