Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation
- PMID: 10323868
- PMCID: PMC316946
- DOI: 10.1101/gad.13.9.1181
Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation
Abstract
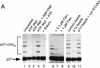
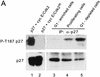
The cellular abundance of the cyclin-dependent kinase (Cdk) inhibitor p27 is regulated by the ubiquitin-proteasome system. Activation of p27 degradation is seen in proliferating cells and in many types of aggressive human carcinomas. p27 can be phosphorylated on threonine 187 by Cdks, and cyclin E/Cdk2 overexpression can stimulate the degradation of wild-type p27, but not of a threonine 187-to-alanine p27 mutant [p27(T187A)]. However, whether threonine 187 phosphorylation stimulates p27 degradation through the ubiquitin-proteasome system or an alternative pathway is still not known. Here, we demonstrate that p27 ubiquitination (as assayed in vivo and in an in vitro reconstituted system) is cell-cycle regulated and that Cdk activity is required for the in vitro ubiquitination of p27. Furthermore, ubiquitination of wild-type p27, but not of p27(T187A), can occur in G1-enriched extracts only upon addition of cyclin E/Cdk2 or cyclin A/Cdk2. Using a phosphothreonine 187 site-specific antibody for p27, we show that threonine 187 phosphorylation of p27 is also cell-cycle dependent, being present in proliferating cells but undetectable in G1 cells. Finally, we show that in addition to threonine 187 phosphorylation, efficient p27 ubiquitination requires formation of a trimeric complex with the cyclin and Cdk subunits. In fact, cyclin B/Cdk1 which can phosphorylate p27 efficiently, but cannot form a stable complex with it, is unable to stimulate p27 ubiquitination by G1 extracts. Furthermore, another p27 mutant [p27(CK-)] that can be phosphorylated by cyclin E/Cdk2 but cannot bind this kinase complex, is refractory to ubiquitination. Thus throughout the cell cycle, both phosphorylation and trimeric complex formation act as signals for the ubiquitination of a Cdk inhibitor.
Figures






References
-
- Alessandrini A, Chiaur DS, Pagano M. Regulation of the cyclin-dependent kinase inhibitor p27 by degradation and phosphorylation. Leukemia. 1997;11:342–345. - PubMed
-
- Amon A, Irniger S, Nasmyth K. Closing the cell cycle circle in yeast: G2 cyclin proteolysis initiated at mitosis persists until the activation of G1 cyclins in the next cycle. Cell. 1994;77:1037–1050. - PubMed
-
- Blain SW, Montalvo E, Massague J. Differential interaction of the cyclin-dependent kinase (Cdk) inhibitor p27Kip1 with cyclin A/Cdk2 and cyclin D2/Cdk4. J Biol Chem. 1997;272:25863–25872. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
