Two modulatory inputs exert reciprocal reinforcing effects on synaptic input of premotor interneurons for withdrawal in terrestrial snails
- PMID: 10327241
- PMCID: PMC311287
Two modulatory inputs exert reciprocal reinforcing effects on synaptic input of premotor interneurons for withdrawal in terrestrial snails
Abstract
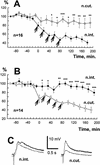
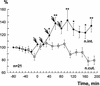
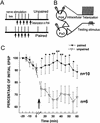
A cluster of serotonergic cells in the rostral part of pedal ganglia of the terrestrial snail Helix lucorum was shown previously to participate in modulation of withdrawal behavior, and to be necessary for elaboration of aversive withdrawal conditioning in intact snails. In the present experiments local extracellular stimulation of the serotonergic cells elicited a pairing-specific increase (difference between paired and explicitly unpaired sessions was significant, P<0.01) of synaptic responses in the premotor interneurons involved in withdrawal to paired nerve stimulation. Intracellular stimulation of only one Pd4 cell from the pedal group of serotonergic neurons increased (P<0.05) synaptic responses to contingent test nerve stimulation significantly in the same premotor interneurons for 2-3 hr. Mesocerebral cells are known to participate in male sexual behavior, and their extracellular stimulation was shown previously to suppress the amplitude of synaptic responses in withdrawal interneurons. Local extracellular stimulation of the mesocerebral cells elicited a pairing-specific decrease (P<0.01) of synaptic responses to contingent test nerve stimulation in the premotor interneurons involved in withdrawal for 2-3 hr. Paired application of met-enkephaline (10(-6) M, some mesocerebral cells are enkephaline-like immunoreactive) also selectively decreased synaptic responses to contingent nerve stimulation in the premotor interneurons for hours. Thus, two modulatory inputs exert pairing-specific effects that influence the same synaptic connection in opposite directions, which may underlie the long-term up- and down-regulation of behavioral responses.
Figures


References
-
- Balaban PM. A system of command neurons in snail’s escape behavior. Acta Neurobiol Exper. 1979;39:97–107. - PubMed
-
- ————— Postsynaptic mechanism of withdrawal reflex sensitization in the snail. J Neurobiol. 1983;14:365–375. - PubMed
-
- ————— . Command neurons, command function and decision making. In: Sakharov DA, Winlow W, editors. Simple nervous systems. Manchester, NY: Manchester University Press; 1991. pp. 360–374.
-
- ———. Behavioral neurobiology of learning in terrestrial snails. Prog. Neurobiol. 41: 1–19. - PubMed
-
- Balaban P, Bravarenko N. Long-term sensitization and environmental conditioning in terrestrial snails. Exp Brain Res. 1993;96:487–493. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
