Decreased proliferation and altered differentiation in osteoblasts from genetically and clinically distinct craniosynostotic disorders
- PMID: 10329600
- PMCID: PMC1866602
- DOI: 10.1016/S0002-9440(10)65401-6
Decreased proliferation and altered differentiation in osteoblasts from genetically and clinically distinct craniosynostotic disorders
Abstract
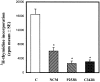
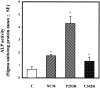
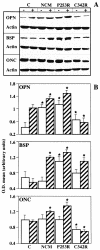
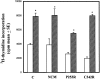
Craniosynostoses are a heterogeneous group of disorders characterized by premature fusion of cranial sutures. Mutations in fibroblast growth factor receptors (FGFRs) have been associated with a number of such conditions. Nevertheless, the cellular mechanism(s) involved remain unknown. We analyzed cell proliferation and differentiation in osteoblasts obtained from patients with three genetically and clinically distinct craniosynostoses: Pfeiffer syndrome carrying the FGFR2 C342R substitution, Apert syndrome with FGFR2 P253R change, and a nonsyndromic craniosynostosis without FGFR canonic mutations, as compared with control osteoblasts. Osteoblasts from craniosynostotic patients exhibited a lower proliferation rate than control osteoblasts. P253R and nonsyndromic craniosynostosis osteoblasts showed a marked differentiated phenotype, characterized by high alkaline phosphatase activity, increased mineralization and expression of noncollagenous matrix proteins, associated with high expression and activation of protein kinase Calpha and protein kinase Cepsilon isoenzymes. By contrast, the low proliferation rate of C342R osteoblasts was not associated with a differentiated phenotype. Although they showed higher alkaline phosphatase activity than control, C342R osteoblasts failed to mineralize and expressed low levels of osteopontin and osteonectin and high protein kinase Czeta levels. Stimulation of proliferation and inhibition of differentiation were observed in all cultures on FGF2 treatment. Our results suggest that an anticipated proliferative/differentiative switch, associated with alterations of the FGFR transduction pathways, could be the causative common feature in craniosynostosis and that mutations in distinct FGFR2 domains are associated with an in vitro heterogeneous differentiative phenotype.
Figures





Similar articles
-
Dura in the pathogenesis of syndromic craniosynostosis: fibroblast growth factor receptor 2 mutations in dural cells promote osteogenic proliferation and differentiation of osteoblasts.J Craniofac Surg. 2010 Mar;21(2):462-7. doi: 10.1097/SCS.0b013e3181cfe9a0. J Craniofac Surg. 2010. PMID: 20489451
-
Apert syndrome mutant FGFR2 and its soluble form reciprocally alter osteogenesis of primary calvarial osteoblasts.J Cell Physiol. 2012 Sep;227(9):3267-77. doi: 10.1002/jcp.24021. J Cell Physiol. 2012. PMID: 22105374
-
Increased calvaria cell differentiation and bone matrix formation induced by fibroblast growth factor receptor 2 mutations in Apert syndrome.J Clin Invest. 1998 Mar 15;101(6):1310-7. J Clin Invest. 1998. PMID: 9502772 Free PMC article.
-
[From gene to disease; craniosynostosis syndromes due to FGFR2-mutation].Ned Tijdschr Geneeskd. 2002 Jan 12;146(2):63-6. Ned Tijdschr Geneeskd. 2002. PMID: 11820058 Review. Dutch.
-
Genetic causes of syndromic craniosynostoses.Eur J Paediatr Neurol. 2013 May;17(3):221-4. doi: 10.1016/j.ejpn.2012.09.009. Epub 2012 Oct 11. Eur J Paediatr Neurol. 2013. PMID: 23062756 Review.
Cited by
-
Differential growth factor adsorption to calvarial osteoblast-secreted extracellular matrices instructs osteoblastic behavior.PLoS One. 2011;6(10):e25990. doi: 10.1371/journal.pone.0025990. Epub 2011 Oct 5. PLoS One. 2011. PMID: 21998741 Free PMC article.
-
Activation of p38 MAPK pathway in the skull abnormalities of Apert syndrome Fgfr2(+P253R) mice.BMC Dev Biol. 2010 Feb 22;10:22. doi: 10.1186/1471-213X-10-22. BMC Dev Biol. 2010. PMID: 20175913 Free PMC article.
-
Understanding craniosynostosis as a growth disorder.Wiley Interdiscip Rev Dev Biol. 2016 Jul;5(4):429-59. doi: 10.1002/wdev.227. Epub 2016 Mar 22. Wiley Interdiscip Rev Dev Biol. 2016. PMID: 27002187 Free PMC article. Review.
-
Characterization of dental pulp stem cells isolated from a patient diagnosed with Crouzon syndrome.J Cell Physiol. 2021 Jul;236(7):5317-5324. doi: 10.1002/jcp.30241. Epub 2021 Jan 1. J Cell Physiol. 2021. PMID: 33386632 Free PMC article.
-
Investigation of FGFR2-IIIC signaling via FGF-2 ligand for advancing GCT stromal cell differentiation.PLoS One. 2012;7(10):e46769. doi: 10.1371/journal.pone.0046769. Epub 2012 Oct 11. PLoS One. 2012. Retraction in: PLoS One. 2019 Jul 23;14(7):e0220356. doi: 10.1371/journal.pone.0220356. PMID: 23071632 Free PMC article. Retracted.
References
-
- Cohen MM, Jr: Craniosynostosis: diagnosis, evaluation and management. 1986. Raven Press, New York
-
- Di Rocco C, Velardi F: Surgical management of craniosynostosis. Galli G eds. Craniosynostosis. 1984, :pp 181-248 CRC Press, Boca Raton, FL
-
- McKusick VA: Mendelian inheritance in man. Catalogs of Human Genes and Genetic Disorders. 1994, Johns Hopkins University Press, Baltimore
-
- Bonaventure J, Rousseau F, Legeai-Mallet L, Le Merrer M, Munnich A, Maroteaux P: Common mutations in the fibroblast growth factor receptor 3 (FGFR3) gene account for achondroplasia, hypochondroplasia, and thanatophoric dwarfism. Am J Med Genet 1996, 63:148-154 - PubMed
-
- Wilkie AOM: Genes and mechanisms. Hum Mol Genet 1997, 6:1647-1656 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

