A human RNA viral cysteine proteinase that depends upon a unique Zn2+-binding finger connecting the two domains of a papain-like fold
- PMID: 10329692
- PMCID: PMC8005983
- DOI: 10.1074/jbc.274.21.14918
A human RNA viral cysteine proteinase that depends upon a unique Zn2+-binding finger connecting the two domains of a papain-like fold
Erratum in
- J Biol Chem 1999 Jul 23;274(30):21490
Abstract
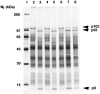
A cysteine proteinase, papain-like proteinase (PL1pro), of the human coronavirus 229E (HCoV) regulates the expression of the replicase polyproteins, pp1a and ppa1ab, by cleavage between Gly111 and Asn112, far upstream of its own catalytic residue Cys1054. In this report, using bioinformatics tools, we predict that, unlike its distant cellular homologues, HCoV PL1pro and its coronaviral relatives have a poorly conserved Zn2+ finger connecting the left and right hand domains of a papain-like fold. Optical emission spectrometry has been used to confirm the presence of Zn2+ in a purified and proteolytically active form of the HCoV PL1pro fused with the Escherichia coli maltose-binding protein. In denaturation/renaturation experiments using the recombinant protein, its activity was shown to be strongly dependent upon Zn2+, which could be partly substituted by Co2+ during renaturation. The reconstituted, Zn2+-containing PL1pro was not sensitive to 1,10-phenanthroline, and the Zn2+-depleted protein was not reactivated by adding Zn2+ after renaturation. Consistent with the proposed essential structural role of Zn2+, PL1pro was selectively inactivated by mutations in the Zn2+ finger, including replacements of any of four conserved Cys residues predicted to co-ordinate Zn2+. The unique domain organization of HCoV PL1pro provides a potential framework for regulatory processes and may be indicative of a nonproteolytic activity of this enzyme.
Figures


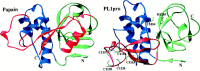
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

