Pattern and kinetics of mouse donor spermatogonial stem cell colonization in recipient testes
- PMID: 10330102
- PMCID: PMC5511737
- DOI: 10.1095/biolreprod60.6.1429
Pattern and kinetics of mouse donor spermatogonial stem cell colonization in recipient testes
Abstract
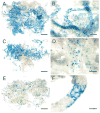
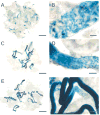
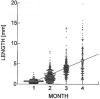
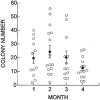
Recently a system was developed in which transplanted donor spermatogonial stem cells establish complete spermatogenesis in the testes of an infertile recipient. To obtain insight into stem cell activity and the behavior of donor germ cells, the pattern and kinetics of mouse spermatogonial colonization in recipient seminiferous tubules were analyzed during the 4 mo following transplantation. The colonization process can be divided into three continuous phases. First, during the initial week, transplanted cells were randomly distributed throughout the tubules, and a small number reached the basement membrane. Second, from 1 wk to 1 mo, donor cells on the basement membrane divided and formed a monolayer network. Third, beginning at about 1 mo and continuing throughout the observation period, cells in the center of the network differentiated extensively and established a colony of spermatogenesis, which expanded laterally by repeating phase two and then three. An average of 19 donor cell-derived colonies developed from 10(6) cells transplanted to the seminiferous tubules of a recipient testis; the number of colonized sites did not change between 1 and 4 mo. However, the length of the colonies increased from 0.73 to 5.78 mm between 1 and 4 mo. These experiments establish the feasibility of studying in a systematic and quantitative manner the pattern and kinetics of the colonization process. Using spermatogonial transplantation as a functional assay, it should be possible to assess the effects of various treatments on stem cells and on recipient seminiferous tubules to provide unique insight into the process of spermatogenesis.
Figures

References
-
- Potten CS. Cell lineages. In: McGee JO'D, Issacson PG, Wright NA, editors. Oxford Textbook of Pathology. Vol. 1. Oxford: Oxford University Press; 1992. pp. 43–52.
-
- Huckins C. The spcrmatogonial stem cell population in adult rats. I. Their morphology, proliferation, and maturation. Anat Rec. 1971;169:533–558. - PubMed
-
- Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–236. - PubMed
-
- Russell LD, Ettlin RA, Sinha Hikim AP, Clcgg ED. Histological and Histopathological Evaluation of the Testis. Clearwater, FL: Cache River Press; 1990. pp. 1–40.
-
- Dym M. The male reproductive system. In: Weiss L, editor. Histology: Cell and Tissue Biology. 5th. New York: Elsevier Science Publishing Co. Inc.; 1983. pp. 1000–1053.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

