Regulation of AUF1 expression via conserved alternatively spliced elements in the 3' untranslated region
- PMID: 10330146
- PMCID: PMC104365
- DOI: 10.1128/MCB.19.6.4056
Regulation of AUF1 expression via conserved alternatively spliced elements in the 3' untranslated region
Abstract
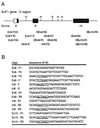
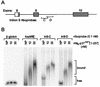
The A+U-rich RNA-binding factor AUF1 exhibits characteristics of a trans-acting factor contributing to the rapid turnover of many cellular mRNAs. Structural mapping of the AUF1 gene and its transcribed mRNA has revealed alternative splicing events within the 3' untranslated region (3'-UTR). In K562 erythroleukemia cells, we have identified four alternatively spliced AUF1 3'-UTR variants, including a population of AUF1 mRNA containing a highly conserved 107-nucleotide (nt) 3'-UTR exon (exon 9) and the adjacent downstream intron (intron 9). Functional analyses using luciferase-AUF1 3'-UTR chimeric transcripts demonstrated that the presence of either a spliceable or an unspliceable intron 9 in the 3'-UTR repressed luciferase expression in cis, indicating that intron 9 sequences may down-regulate gene expression by two distinct mechanisms. In the case of the unspliceable intron, repression of luciferase expression likely involved two AUF1-binding sequences, since luciferase expression was increased by deletion of these sites. However, inclusion of the spliceable intron in the luciferase 3'-UTR down-regulated expression independent of the AUF1-binding sequences. This is likely due to nonsense-mediated mRNA decay (NMD) owing to the generation of exon-exon junctions more than 50 nt downstream of the luciferase termination codon. AUF1 mRNA splice variants generated by selective excision of intron 9 are thus also likely to be subject to NMD since intron 9 is always positioned >137 nt downstream of the stop codon. The distribution of alternatively spliced AUF1 transcripts in K562 cells is consistent with this model of regulated AUF1 expression.
Figures








References
-
- Atwater J A, Wisdom R, Verma I M. Regulated mRNA stability. Annu Rev Genet. 1990;24:519–541. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
