Transcriptional repressor ERF is a Ras/mitogen-activated protein kinase target that regulates cellular proliferation
- PMID: 10330152
- PMCID: PMC104371
- DOI: 10.1128/MCB.19.6.4121
Transcriptional repressor ERF is a Ras/mitogen-activated protein kinase target that regulates cellular proliferation
Abstract
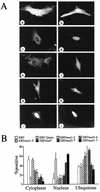
A limited number of transcription factors have been suggested to be regulated directly by Erks within the Ras/mitogen-activated protein kinase signaling pathway. In this paper we demonstrate that ERF, a ubiquitously expressed transcriptional repressor that belongs to the Ets family, is physically associated with and phosphorylated in vitro and in vivo by Erks. This phosphorylation determines the ERF subcellular localization. Upon mitogenic stimulation, ERF is immediately phosphorylated and exported to the cytoplasm. The export is blocked by specific Erk inhibitors and is abolished when residues undergoing phosphorylation are mutated to alanine. Upon growth factor deprivation, ERF is rapidly dephosphorylated and transported back into the nucleus. Phosphorylation-defective ERF mutations suppress Ras-induced tumorigenicity and arrest the cells at the G0/G1 phase of the cell cycle. Our findings strongly suggest that ERF may be important in the control of cellular proliferation during the G0/G1 transition and that it may be one of the effectors in the mammalian Ras signaling pathway.
Figures
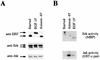





References
-
- Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell R G. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. - PubMed
-
- Brunner D, Ducker K, Oellers N, Hafen E, Scholz H, Klambt C. The ETS domain protein pointed-P2 is a target of MAP kinase in the sevenless signal transduction pathway. Nature. 1994;370:386–389. - PubMed
-
- Chow C W, Rincon M, Cavanagh J, Dickens M, Davis R J. Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science. 1997;278:1638–1641. - PubMed
-
- Coso O A, Chiariello M, Yu J C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
