Platelet-derived growth factor-stimulated expression of the MCP-1 immediate-early gene involves an inhibitory multiprotein complex
- PMID: 10330162
- PMCID: PMC104381
- DOI: 10.1128/MCB.19.6.4219
Platelet-derived growth factor-stimulated expression of the MCP-1 immediate-early gene involves an inhibitory multiprotein complex
Abstract
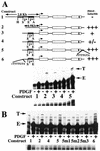
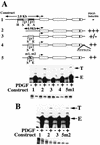
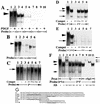
We have demonstrated previously that the seven-nucleotide (nt) motif TTTTGTA (the heptamer) that is present within the proximal 3' untranslated sequences of numerous immediate-early genes is essential for platelet-derived growth factor (PDGF)-stimulated induction of the MCP-1 immediate-early gene. On this basis, the heptamer was suggested to be a conserved regulatory element involved in immediate-early gene expression, although its mechanism of action was unknown. Herein, we demonstrate that the heptamer functions to remove an inhibition of PDGF induction of MCP-1 maintained by two independently acting inhibitory elements present in the MCP-1 5' flanking sequences (designated I* elements). PDGF treatment relieves the I*-mediated inhibition of MCP-1 expression only if the heptamer is also present. One inhibitory element is contained within a 59-nt portion of MCP-1 5' flanking sequences and functions in an orientation-independent and heptamer-regulated manner. Significantly, proteins binding to two DNA sequences contribute to the formation of a single multiprotein complex on the 59-nt I* element. The I*-binding complex contains Sp3, an Sp1-like protein, and a novel DNA-binding protein. Moreover, the complex does not form on two 59-nt sequences containing mutations that reverse the inhibition of PDGF induction maintained by the wild-type I* element. We propose to call the multiprotein I*-binding complex a repressosome and suggest that it acts to repress PDGF-stimulated transcription of MCP-1 in the absence of the heptamer TTTTGTA.
Figures





Similar articles
-
trans-Retinoic acid blocks platelet-derived growth factor-BB-induced expression of the murine monocyte chemoattractant-1 gene by blocking the assembly of a promoter proximal Sp1 binding site.J Biol Chem. 1999 Nov 5;274(45):31909-16. doi: 10.1074/jbc.274.45.31909. J Biol Chem. 1999. PMID: 10542218
-
A new platelet-derived growth factor-regulated genomic element which binds a serine/threonine phosphoprotein mediates induction of the slow immediate-early gene MCP-1.Mol Cell Biol. 1995 Jan;15(1):315-25. doi: 10.1128/MCB.15.1.315. Mol Cell Biol. 1995. PMID: 7799939 Free PMC article.
-
Platelet-derived growth factor induction of the immediate-early gene MCP-1 is mediated by NF-kappaB and a 90-kDa phosphoprotein coactivator.J Biol Chem. 1996 Jul 19;271(29):17417-24. doi: 10.1074/jbc.271.29.17417. J Biol Chem. 1996. PMID: 8663287
-
Regulation of the integrin subunit alpha5 gene promoter by the transcription factors Sp1/Sp3 is influenced by the cell density in rabbit corneal epithelial cells.Invest Ophthalmol Vis Sci. 2003 Sep;44(9):3742-55. doi: 10.1167/iovs.03-0191. Invest Ophthalmol Vis Sci. 2003. PMID: 12939287
-
A novel 7-nucleotide motif located in 3' untranslated sequences of the immediate-early gene set mediates platelet-derived growth factor induction of the JE gene.Mol Cell Biol. 1992 Dec;12(12):5288-300. doi: 10.1128/mcb.12.12.5288-5300.1992. Mol Cell Biol. 1992. PMID: 1448065 Free PMC article.
Cited by
-
Sp1 regulates chromatin looping between an intronic enhancer and distal promoter of the human heme oxygenase-1 gene in renal cells.J Biol Chem. 2010 May 28;285(22):16476-86. doi: 10.1074/jbc.M109.058586. Epub 2010 Mar 29. J Biol Chem. 2010. PMID: 20351094 Free PMC article.
-
Aqueous humor cytokine levels through microarray analysis and a sub-analysis based on optical coherence tomography in wet age-related macular degeneration patients.BMC Ophthalmol. 2021 Nov 18;21(1):399. doi: 10.1186/s12886-021-02152-6. BMC Ophthalmol. 2021. PMID: 34794403 Free PMC article.
-
Molecular mechanism(s) involved in the synergistic induction of CXCL10 by human immunodeficiency virus type 1 Tat and interferon-gamma in macrophages.J Neurovirol. 2008 May;14(3):196-204. doi: 10.1080/13550280801993648. J Neurovirol. 2008. PMID: 18569454 Free PMC article.
References
-
- Ayer D E, Eisenman R N. A switch from Myc:Max to Mad:Max heterocomplexes accompanies monocyte/macrophage differentiation. Genes Dev. 1993;7:2110–2119. - PubMed
-
- Bigger C B, Melnikova I N, Gardner P D. Sp1 and Sp3 regulate expression of the neuronal nicotinic acetylcholine receptor beta-4 subunit gene. J Biol Chem. 1997;272:25976–25982. - PubMed
-
- Boring L, Gosling J, Cleary M, Charo I F. Decreased lesion formation in CCR2 −/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
