Structural and functional heterogeneity of nuclear bodies
- PMID: 10330182
- PMCID: PMC104401
- DOI: 10.1128/MCB.19.6.4423
Structural and functional heterogeneity of nuclear bodies
Abstract
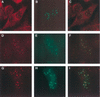
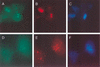
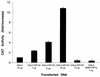
The nuclear body is a cellular structure that appears to be involved in the pathogenesis of acute promyelocytic leukemia and viral infection. In addition, the nuclear body is a target of autoantibodies in patients with the autoimmune disease primary biliary cirrhosis. Although the precise function of the nuclear body in normal cellular biology is unknown, this structure may have a role in the regulation of gene transcription. In a previous investigation, we identified a leukocyte-specific, gamma interferon (IFN-gamma)-inducible autoantigen designated Sp140. The objectives of the present study were to investigate the cellular location of Sp140 with respect to the nuclear-body components PML and Sp100 and to examine the potential role of Sp140 in the regulation of gene transcription. We used adenovirus-mediated gene transfer to express Sp140 in human cells and observed that the protein colocalized with PML and Sp100 in resting cells and associated with structures containing PML during mitosis. In cells infected with the adenovirus expressing Sp140 and incubated with IFN-gamma, the number of PML-Sp100 nuclear bodies per cell increased but immunoreactive Sp140 was not evenly distributed among the nuclear bodies. Sp140 associated with a subset of IFN-gamma-induced PML-Sp100 nuclear bodies. To examine the potential effect of Sp140 on gene transcription, a plasmid encoding Sp140 fused to the DNA-binding domain of GAL4 was cotransfected into COS cells with a chloramphenicol acetyltransferase (CAT) reporter gene containing five GAL4-binding sites and a simian virus 40 enhancer region. The GAL4-Sp140 fusion protein increased the expression of the reporter gene. In contrast, Sp100 fused to the GAL4 DNA-binding domain inhibited CAT activity in transfected mammalian cells. The results of this study demonstrate that Sp140 associates with a subset of PML-Sp100 nuclear bodies in IFN-gamma-treated cells and that Sp140 may activate gene transcription. Taken together, these observations suggest that the nuclear bodies within a cell may be heterogeneous with respect to both composition and function.
Figures






References
-
- Ahn M-J, Nason-Burchenal K, Moasser M M, Dmitrovsky E. Growth suppression of acute promyelocytic leukemia cells having increased expression of the non-rearranged alleles; RARα or PML. Oncogene. 1995;10:2307–2314. - PubMed
-
- Bloch D B, de la Monte S M, Guigaouri P, Filippov A, Bloch K D. Identification and characterization of a leukocyte-specific component of the nuclear body. J Biol Chem. 1996;46:29198–29204. - PubMed
-
- Borrow J, Goddard A, Sheer D, Solomon E. Molecular analysis of acute promyelocytic leukemia breakpoint cluster region on chromosome 17. Science. 1990;249:1577–1580. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
