Yeast VSM1 encodes a v-SNARE binding protein that may act as a negative regulator of constitutive exocytosis
- PMID: 10330187
- PMCID: PMC104406
- DOI: 10.1128/MCB.19.6.4480
Yeast VSM1 encodes a v-SNARE binding protein that may act as a negative regulator of constitutive exocytosis
Erratum in
- Mol Cell Biol. 2008 Dec;28(24):7534
Abstract
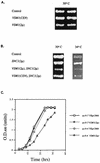
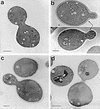
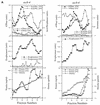
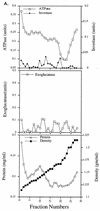
We have screened for proteins that interact with v-SNAREs of the late secretory pathway in the yeast Saccharomyces cerevisiae. A novel protein, designated Vsm1, binds tightly to the Snc2 v-SNARE in the two-hybrid system and can be coimmunoprecipitated with Snc1 or Snc2 from solubilized yeast cell extracts. Disruption of the VSM1 gene results in an increase of proteins secreted into the medium but does not affect the processing or secretion of invertase. In contrast, VSM1 overexpression in cells which bear a temperature-sensitive mutation in the Sec9 t-SNARE (sec9-4 cells) results in the accumulation of non-invertase-containing low-density secretory vesicles, inhibits cell growth and the secretion of proteins into the medium, and blocks rescue of the temperature-sensitive phenotype by SNC1 overexpression. Yet, VSM1 overexpression does not affect yeast bearing a sec9-7 allele which, in contrast to sec9-4, encodes a t-SNARE protein capable of forming a stable SNARE complex in vitro at restrictive temperatures. On the basis of these results, we propose that Vsm1 is a novel v-SNARE-interacting protein that appears to act as negative regulator of constitutive exocytosis. Moreover, this regulation appears specific to one of two parallel exocytic paths which are operant in yeast cells.
Figures





References
-
- Aalto M K, Ruohonen L, Hosono K, Keranen S. Cloning and sequencing of the yeast Saccharomyces cerevisiae SEC1 gene localized on chromosome IV. Yeast. 1992;8:587–588. - PubMed
-
- Ames B N. Assay of inorganic phosphate, total phosphate, and phosphatases. Methods Enzymol. 1966;8:115–118.
-
- Araki S, Tamori Y, Kawanishi M, Shinoda H, Masugi J, Mori H, Niki T, Okazawa H, Kubota T, Kasuga M. Inhibition of the binding of SNAP-23 to syntaxin 4 by Munc18c. Biochem Biophys Res Commun. 1997;234:257–262. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
