LST1 is a SEC24 homologue used for selective export of the plasma membrane ATPase from the endoplasmic reticulum
- PMID: 10330397
- PMCID: PMC2133178
- DOI: 10.1083/jcb.145.4.659
LST1 is a SEC24 homologue used for selective export of the plasma membrane ATPase from the endoplasmic reticulum
Abstract
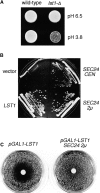
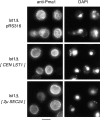
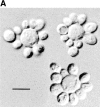
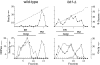
In Saccharomyces cerevisiae, vesicles that carry proteins from the ER to the Golgi compartment are encapsulated by COPII coat proteins. We identified mutations in ten genes, designated LST (lethal with sec-thirteen), that were lethal in combination with the COPII mutation sec13-1. LST1 showed synthetic-lethal interactions with the complete set of COPII genes, indicating that LST1 encodes a new COPII function. LST1 codes for a protein similar in sequence to the COPII subunit Sec24p. Like Sec24p, Lst1p is a peripheral ER membrane protein that binds to the COPII subunit Sec23p. Chromosomal deletion of LST1 is not lethal, but inhibits transport of the plasma membrane proton-ATPase (Pma1p) to the cell surface, causing poor growth on media of low pH. Localization by both immunofluorescence microscopy and cell fractionation shows that the export of Pma1p from the ER is impaired in lst1Delta mutants. Transport of other proteins from the ER was not affected by lst1Delta, nor was Pma1p transport found to be particularly sensitive to other COPII defects. Together, these findings suggest that a specialized form of the COPII coat subunit, with Lst1p in place of Sec24p, is used for the efficient packaging of Pma1p into vesicles derived from the ER.
Figures






References
-
- Barlowe C, Schekman R. SEC12encodes a guanine-nucleotide- exchange factor essential for transport vesicle budding from the ER. Nature. 1993;365:347–349. - PubMed
-
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. - PubMed
-
- Bartel PL, Fields S. Analyzing protein–protein interactions using two-hybrid system. Methods Enzymol. 1995;254:241–263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

