Fusogenic domains of golgi membranes are sequestered into specialized regions of the stack that can be released by mechanical fragmentation
- PMID: 10330398
- PMCID: PMC2133190
- DOI: 10.1083/jcb.145.4.673
Fusogenic domains of golgi membranes are sequestered into specialized regions of the stack that can be released by mechanical fragmentation
Abstract
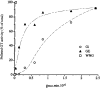
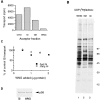
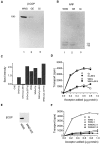
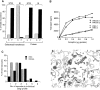
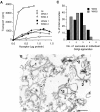
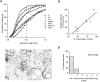
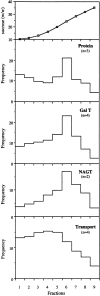
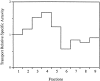
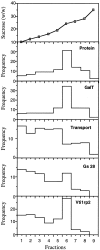
A well-characterized cell-free assay that reconstitutes Golgi transport is shown to require physically fragmented Golgi fractions for maximal activity. A Golgi fraction containing large, highly stacked flattened cisternae associated with coatomer-rich components was inactive in the intra-Golgi transport assay. In contrast, more fragmented hepatic Golgi fractions of lower purity were highly active in this assay. Control experiments ruled out defects in glycosylation, the presence of excess coatomer or inhibitory factors, as well as the lack or consumption of limiting diffusible factors as responsible for the lower activity of intact Golgi fractions. Neither Brefeldin A treatment, preincubation with KCl (that completely removed associated coatomer) or preincubation with imidazole buffers that caused unstacking, activated stacked fractions for transport. Only physical fragmentation promoted recovery of Golgi fractions active for transport in vitro. Rate-zonal centrifugation partially separated smaller transport-active Golgi fragments with a unique v-SNARE pattern, away from the bulk of Golgi-derived elements identified by their morphology and content of Golgi marker enzymes (N-acetyl glucosaminyl and galactosyl transferase activities). These fragments released during activation likely represent intra-Golgi continuities involved in maintaining the dynamic redistribution of resident enzymes during rapid anterograde transport of secretory cargo through the Golgi in vivo.
Figures




References
-
- Acharya U, Jacobs R, Peters J-M, Watson N, Farquhar MG, Malhotra V. The formation of Golgi stacks from vesiculated Golgi membranes requires two distinct fusion events. Cell. 1995;82:895–904. - PubMed
-
- Balch WE, Dunphy WG, Braell WA, Rothman JE. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984;39:405–416. - PubMed
-
- Balch WE, Rothman JE. Characterization of protein transport between successive compartments of the Golgi apparatus: asymmetric properties of donor and acceptor activities in a cell-free system. Arch Biochem Biophys. 1985;240:413–425. - PubMed

