Overexpression of protein kinase C betaII induces colonic hyperproliferation and increased sensitivity to colon carcinogenesis
- PMID: 10330400
- PMCID: PMC2133187
- DOI: 10.1083/jcb.145.4.699
Overexpression of protein kinase C betaII induces colonic hyperproliferation and increased sensitivity to colon carcinogenesis
Abstract
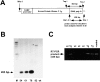
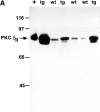
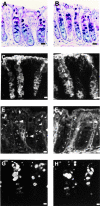
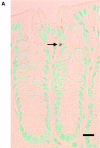
Protein kinase C betaII (PKC betaII) has been implicated in proliferation of the intestinal epithelium. To investigate PKC betaII function in vivo, we generated transgenic mice that overexpress PKC betaII in the intestinal epithelium. Transgenic PKC betaII mice exhibit hyperproliferation of the colonic epithelium and an increased susceptibility to azoxymethane-induced aberrant crypt foci, preneoplastic lesions in the colon. Furthermore, transgenic PKC betaII mice exhibit elevated colonic beta-catenin levels and decreased glycogen synthase kinase 3beta activity, indicating that PKC betaII stimulates the Wnt/adenomatous polyposis coli (APC)/beta-catenin proliferative signaling pathway in vivo. These data demonstrate a direct role for PKC betaII in colonic epithelial cell proliferation and colon carcinogenesis, possibly through activation of the APC/beta-catenin signaling pathway.
Figures








References
-
- Bertagnolli MM, McDougall CJ, Newmark HL. Colon cancer prevention: intervening in a multistage process. Proc Soc Exp Biol Med. 1997;216:266–274. - PubMed
-
- Bird RP. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 1987;37:147–151. - PubMed
-
- Bird RP. Role of aberrant crypt foci in understanding the pathogenesis of colon cancer. Cancer Lett. 1995;93:55–71. - PubMed
-
- Boland CR, Ahnen DJ. Binding of lectins to goblet cell mucin in malignant and premalignant colonic epithelium in the CF-1 mouse. Gastroenterology. 1995;89:127–137. - PubMed
-
- Caldero J, Campo E, Vinas J, Cardesa A. Lectin-binding sites in neoplastic and non-neoplastic colonic mucosa of 1,2-dimethylhydrazine-treated rats. Lab Invest. 1989;61:670–676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

