Changes in the balance of phosphoinositide 3-kinase/protein kinase B (Akt) and the mitogen-activated protein kinases (ERK/p38MAPK) determine a phenotype of visceral and vascular smooth muscle cells
- PMID: 10330402
- PMCID: PMC2133182
- DOI: 10.1083/jcb.145.4.727
Changes in the balance of phosphoinositide 3-kinase/protein kinase B (Akt) and the mitogen-activated protein kinases (ERK/p38MAPK) determine a phenotype of visceral and vascular smooth muscle cells
Abstract
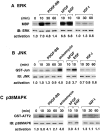
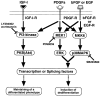
The molecular mechanisms behind phenotypic modulation of smooth muscle cells (SMCs) remain unclear. In our recent paper, we reported the establishment of novel culture system of gizzard SMCs (Hayashi, K., H. Saga, Y. Chimori, K. Kimura, Y. Yamanaka, and K. Sobue. 1998. J. Biol. Chem. 273: 28860-28867), in which insulin-like growth factor-I (IGF-I) was the most potent for maintaining the differentiated SMC phenotype, and IGF-I triggered the phosphoinositide 3-kinase (PI3-K) and protein kinase B (PKB(Akt)) pathway. Here, we investigated the signaling pathways involved in de-differentiation of gizzard SMCs induced by PDGF-BB, bFGF, and EGF. In contrast to the IGF-I-triggered pathway, PDGF-BB, bFGF, and EGF coordinately activated ERK and p38MAPK pathways. Further, the forced expression of active forms of MEK1 and MKK6, which are the upstream kinases of ERK and p38MAPK, respectively, induced de-differentiation even when SMCs were stimulated with IGF-I. Among three growth factors, PDGF-BB only triggered the PI3-K/PKB(Akt) pathway in addition to the ERK and p38MAPK pathways. When the ERK and p38MAPK pathways were simultaneously blocked by their specific inhibitors or an active form of either PI3-K or PKB(Akt) was transfected, PDGF-BB in turn initiated to maintain the differentiated SMC phenotype. We applied these findings to vascular SMCs, and demonstrated the possibility that the same signaling pathways might be involved in regulating the vascular SMC phenotype. These results suggest that changes in the balance between the PI3-K/PKB(Akt) pathway and the ERK and p38MAPK pathways would determine phenotypes of visceral and vascular SMCs. We further reported that SMCs cotransfected with active forms of MEK1 and MKK6 secreted a nondialyzable, heat-labile protein factor(s) which induced de-differentiation of surrounding normal SMCs.
Figures













Similar articles
-
Dual control of muscle cell survival by distinct growth factor-regulated signaling pathways.Mol Cell Biol. 2000 May;20(9):3256-65. doi: 10.1128/MCB.20.9.3256-3265.2000. Mol Cell Biol. 2000. PMID: 10757809 Free PMC article.
-
Platelet-derived growth factor-BB, insulin-like growth factor-I, and phorbol ester activate different signaling pathways for stimulation of vascular smooth muscle cell migration.Exp Cell Res. 1998 Aug 1;242(2):548-60. doi: 10.1006/excr.1998.4138. Exp Cell Res. 1998. PMID: 9683541
-
The cell survival signal Akt is differentially activated by PDGF-BB, EGF, and FGF-2 in osteoblastic cells.J Cell Biochem. 2001 Mar 26;81(2):304-11. J Cell Biochem. 2001. PMID: 11241670
-
Signaling through PI3K/Akt mediates stretch and PDGF-BB-dependent DNA synthesis in bladder smooth muscle cells.J Urol. 2003 Jun;169(6):2388-93. doi: 10.1097/01.ju.0000063980.99368.35. J Urol. 2003. PMID: 12771803
-
The role of tribbles homolog 2 in cell proliferation.Cell Commun Signal. 2025 Jan 6;23(1):5. doi: 10.1186/s12964-024-01985-0. Cell Commun Signal. 2025. PMID: 39762856 Free PMC article. Review.
Cited by
-
Muscarinic M(3) receptor-dependent regulation of airway smooth muscle contractile phenotype.Br J Pharmacol. 2004 Mar;141(6):943-50. doi: 10.1038/sj.bjp.0705709. Epub 2004 Mar 1. Br J Pharmacol. 2004. PMID: 14993104 Free PMC article.
-
Rapamycin Treatment Attenuates Angiotensin II -induced Abdominal Aortic Aneurysm Formation via VSMC Phenotypic Modulation and Down-regulation of ERK1/2 Activity.Curr Med Sci. 2018 Feb;38(1):93-100. doi: 10.1007/s11596-018-1851-z. Epub 2018 Mar 15. Curr Med Sci. 2018. PMID: 30074157
-
Angiotensin type I receptor blockade in conjunction with enhanced Akt activation restores coronary collateral growth in the metabolic syndrome.Am J Physiol Heart Circ Physiol. 2011 May;300(5):H1938-49. doi: 10.1152/ajpheart.00282.2010. Epub 2011 Feb 18. Am J Physiol Heart Circ Physiol. 2011. PMID: 21335466 Free PMC article.
-
Cardiac epithelial-mesenchymal transition is blocked by monomethylarsonous acid (III).Toxicol Sci. 2014 Nov;142(1):225-38. doi: 10.1093/toxsci/kfu170. Epub 2014 Aug 21. Toxicol Sci. 2014. PMID: 25145660 Free PMC article.
-
Integrin-linked kinase regulates smooth muscle differentiation marker gene expression in airway tissue.Am J Physiol Lung Cell Mol Physiol. 2008 Dec;295(6):L988-97. doi: 10.1152/ajplung.90202.2008. Epub 2008 Sep 19. Am J Physiol Lung Cell Mol Physiol. 2008. PMID: 18805960 Free PMC article.
References
-
- Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. - PubMed
-
- Bornfeldt KE, Arnqvist HL, Norstedt G. Regulation of insulin-like growth factor-I gene expression by growth factors in cultured vascular smooth muscle cells. J Endocrinol. 1990;125:381–386. - PubMed
-
- Bornfeldt KE, Rainco EW, Nateano T, Groves LM, Krebs E, Ross R. Insulin-like growth factor-I and platelet-derived growth factor-BB induce directed migration of human arterial smooth muscle cells via signaling pathways that are distinct from those of proliferation. J Clin Invest. 1994;93:1266–1274. - PMC - PubMed
-
- Cercek B, Fishbein MC, Forrester JS, Helfant RH, Fagin JA. Induction of insulin-like growth factor I messenger RNA in rat aorta after balloon denudation. Circ Res. 1990;66:1755–1760. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

