Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes
- PMID: 10330407
- PMCID: PMC2133193
- DOI: 10.1083/jcb.145.4.795
Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes
Abstract
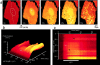
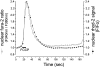
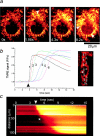
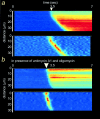
We have used digital fluorescence imaging techniques to explore the interplay between mitochondrial Ca2+ uptake and physiological Ca2+ signaling in rat cortical astrocytes. A rise in cytosolic Ca2+ ([Ca2+]cyt), resulting from mobilization of ER Ca2+ stores was followed by a rise in mitochondrial Ca2+ ([Ca2+]m, monitored using rhod-2). Whereas [Ca2+]cyt recovered within approximately 1 min, the time to recovery for [Ca2+]m was approximately 30 min. Dissipating the mitochondrial membrane potential (Deltapsim, using the mitochondrial uncoupler carbonyl cyanide p-trifluoromethoxy-phenyl-hydrazone [FCCP] with oligomycin) prevented mitochondrial Ca2+ uptake and slowed the rate of decay of [Ca2+]cyt transients, suggesting that mitochondrial Ca2+ uptake plays a significant role in the clearance of physiological [Ca2+]cyt loads in astrocytes. Ca2+ signals in these cells initiated either by receptor-mediated ER Ca2+ release or mechanical stimulation often consisted of propagating waves (measured using fluo-3). In response to either stimulus, the wave traveled at a mean speed of 22.9 +/- 11.2 micrometer/s (n = 262). This was followed by a wave of mitochondrial depolarization (measured using tetramethylrhodamine ethyl ester [TMRE]), consistent with Ca2+ uptake into mitochondria as the Ca2+ wave traveled across the cell. Collapse of Deltapsim to prevent mitochondrial Ca2+ uptake significantly increased the rate of propagation of the Ca2+ waves by 50%. Taken together, these data suggest that cytosolic Ca2+ buffering by mitochondria provides a potent mechanism to regulate the localized spread of astrocytic Ca2+ signals.
Figures




References
-
- al Mohanna, F.A., K.W. Caddy, and S.R. Bolsover. The nucleus is insulated from large cytosolic calcium ion changes. Nature. 1994;367:745–750. - PubMed
-
- Bernardi P, Broekemeier KM, Pfeiffer DR. Recent progress on regulation of the mitochondrial permeability transition pore, a cyclosporin-sensitive pore in the inner mitochondrial membrane. J Bioenerg Biomembr. 1994;26:509–517. - PubMed
-
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. - PubMed
-
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

