Localization of core spindle pole body (SPB) components during SPB duplication in Saccharomyces cerevisiae
- PMID: 10330408
- PMCID: PMC2133189
- DOI: 10.1083/jcb.145.4.809
Localization of core spindle pole body (SPB) components during SPB duplication in Saccharomyces cerevisiae
Abstract
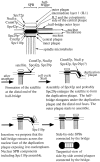
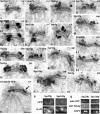
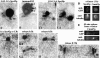
We have examined the process of spindle pole body (SPB) duplication in Saccharomyces cerevisiae by electron microscopy and found several stages. These include the assembly, probably from the satellite, of a large plaque-like structure, the duplication plaque, on the cytoplasmic face of the half-bridge and its insertion into the nuclear envelope. We analyzed the role of the main SPB components in the formation of these structures by identifying them from an SPB core fraction by mass spectrometry. Temperature-sensitive mutants for two of the components, Spc29p and Nud1p, were prepared to partly define their function. The composition of two of the intermediates in SPB duplication, the satellite and the duplication plaque, was examined by immunoelectron microscopy. Both contain cytoplasmic SPB components showing that duplication has already been partly achieved by the end of the preceding cell cycle when the satellite is formed. We show that by overexpression of SPB components the structure of the satellite can be changed and SPB duplication inhibited by disrupting the attachment of the plaque-like intermediate to the half-bridge. We present a model for SPB duplication where binding of SPB components to either end of the bridge structure ensures two separate SPBs.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

