Neurotractin, a novel neurite outgrowth-promoting Ig-like protein that interacts with CEPU-1 and LAMP
- PMID: 10330412
- PMCID: PMC2133198
- DOI: 10.1083/jcb.145.4.865
Neurotractin, a novel neurite outgrowth-promoting Ig-like protein that interacts with CEPU-1 and LAMP
Abstract
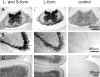
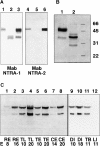
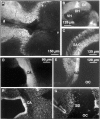
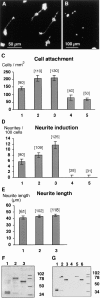
The formation of axon tracts in nervous system histogenesis is the result of selective axon fasciculation and specific growth cone guidance in embryonic development. One group of proteins implicated in neurite outgrowth, fasciculation, and guidance is the neural members of the Ig superfamily (IgSF). In an attempt to identify and characterize new proteins of this superfamily in the developing nervous system, we used a PCR-based strategy with degenerated primers that represent conserved sequences around the characteristic cysteine residues of Ig-like domains. Using this approach, we identified a novel neural IgSF member, termed neurotractin. This GPI-linked cell surface glycoprotein is composed of three Ig-like domains and belongs to the IgLON subgroup of neural IgSF members. It is expressed in two isoforms with apparent molecular masses of 50 and 37 kD, termed L-form and S-form, respectively. Monoclonal antibodies were used to analyze its biochemical features and histological distribution. Neurotractin is restricted to subsets of developing commissural and longitudinal axon tracts in the chick central nervous system. Recombinant neurotractin promotes neurite outgrowth of telencephalic neurons and interacts with the IgSF members CEPU-1 (KD = 3 x 10(-8) M) and LAMP. Our data suggest that neurotractin participates in the regulation of neurite outgrowth in the developing brain.
Figures


Similar articles
-
A family of glycoproteins (GP55), which inhibit neurite outgrowth, are members of the Ig superfamily and are related to OBCAM, neurotrimin, LAMP and CEPU-1.J Cell Sci. 1996 Dec;109 ( Pt 13):3129-38. doi: 10.1242/jcs.109.13.3129. J Cell Sci. 1996. PMID: 9004047
-
Promotion of neuronal cell adhesion by members of the IgLON family occurs in the absence of either support or modification of neurite outgrowth.J Neurochem. 2002 Mar;80(6):941-8. doi: 10.1046/j.0022-3042.2002.00798.x. J Neurochem. 2002. PMID: 11953444
-
Co-localisation, heterophilic interactions and regulated expression of IgLON family proteins in the chick nervous system.Brain Res Mol Brain Res. 2000 Oct 20;82(1-2):84-94. doi: 10.1016/s0169-328x(00)00184-4. Brain Res Mol Brain Res. 2000. PMID: 11042360
-
Glycosylphosphatidylinositol anchored recognition molecules that function in axonal fasciculation, growth and guidance in the nervous system.Cell Biol Int Rep. 1991 Nov;15(11):1151-66. doi: 10.1016/0309-1651(91)90061-m. Cell Biol Int Rep. 1991. PMID: 1838307 Review.
-
Tenascin-contactin/F11 interactions: a clue for a developmental role?Perspect Dev Neurobiol. 1994;2(1):43-52. Perspect Dev Neurobiol. 1994. PMID: 7530143 Review.
Cited by
-
Obesity genetics in mouse and human: back and forth, and back again.PeerJ. 2015 Mar 24;3:e856. doi: 10.7717/peerj.856. eCollection 2015. PeerJ. 2015. PMID: 25825681 Free PMC article. Review.
-
Genome-wide association study of smoking trajectory and meta-analysis of smoking status in 842,000 individuals.Nat Commun. 2020 Oct 20;11(1):5302. doi: 10.1038/s41467-020-18489-3. Nat Commun. 2020. PMID: 33082346 Free PMC article.
-
Expression of fourteen novel obesity-related genes in Zucker diabetic fatty rats.Cardiovasc Diabetol. 2012 Jul 13;11:48. doi: 10.1186/1475-2840-11-48. Cardiovasc Diabetol. 2012. PMID: 22553958 Free PMC article.
-
In silico approach to identify non-synonymous SNPs with highest predicted deleterious effect on protein function in human obesity related gene, neuronal growth regulator 1 (NEGR1).3 Biotech. 2018 Nov;8(11):466. doi: 10.1007/s13205-018-1463-0. Epub 2018 Nov 1. 3 Biotech. 2018. PMID: 30402368 Free PMC article.
-
Six new loci associated with body mass index highlight a neuronal influence on body weight regulation.Nat Genet. 2009 Jan;41(1):25-34. doi: 10.1038/ng.287. Epub 2008 Dec 14. Nat Genet. 2009. PMID: 19079261 Free PMC article.
References
-
- Bisswanger, H. 1979. Theorie und Methoden der Enzymkinetik. Verlag Chemie, Weinheim.
-
- Brümmendorf T, Spaltmann F, Treubert U. Cloning and characterization of a neural cell recognition molecule on axons of the retinotectal system and spinal cord. Eur J Neurosci. 1997;9:1105–1116. - PubMed
-
- Brümmendorf T, Kenwrick S, Rathjen FG. Neural cell recognition molecule L1: from cell biology to human hereditary brain malformations. Curr Opin Neurobiol. 1998;8:87–97. - PubMed
-
- Brümmendorf T, Rathjen FG. Cell adhesion molecules 1: immunoglobulin superfamily. Prot Profile. 1995;2:963–1108. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

