Identification of a contractile deficit in adult cardiac myocytes expressing hypertrophic cardiomyopathy-associated mutant troponin T proteins
- PMID: 10330428
- PMCID: PMC408456
- DOI: 10.1172/JCI6377
Identification of a contractile deficit in adult cardiac myocytes expressing hypertrophic cardiomyopathy-associated mutant troponin T proteins
Abstract
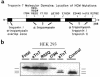
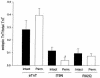
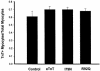
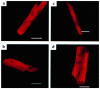
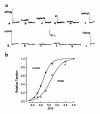
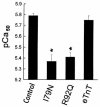
The direct effects of expressing hypertrophic cardiomyopathy-associated (HCM-associated) mutant troponin T (TnT) proteins on the force generation of single adult cardiac myocytes have not been established. Replication-defective recombinant adenovirus vectors were generated for gene transfer of HCM-associated I79N and R92Q mutant cardiac TnT cDNAs into fully differentiated adult cardiac myocytes in primary culture. We tested the hypothesis that the mutant TnT proteins would be expressed and incorporated into the cardiac sarcomere and would behave as dominant-negative proteins to directly alter calcium-activated force generation at the level of the single cardiac myocyte. Interestingly, under identical experimental conditions, the ectopic expression of the mutant TnTs was significantly less ( approximately 8% of total) than that obtained with expression of wild-type TnT ( approximately 35%) in the myocytes. Confocal imaging of immunolabeled TnT showed a regular periodic pattern of localization of ectopic mutant TnT that was not different than that in normal controls, suggesting that mutant TnT incorporation had no deleterious effects on sarcomeric architecture. Direct measurements of isometric force production in single cardiac myocytes demonstrated marked desensitization of submaximal calcium-activated tension, with unchanged maximum tension generation in mutant TnT-expressing myocytes compared with control myocytes. Collectively, these results demonstrate an impaired expression of the mutant protein and a disabling of cardiac contraction in the submaximal range of myoplasmic calcium concentrations. Our functional results suggest that development of new pharmacological, chemical, or genetic approaches to sensitize the thin-filament regulatory protein system could ameliorate force deficits associated with expression of I79N and R92Q in adult cardiac myocytes.
Figures



References
-
- Bonne G, Carrier L, Richard P, Hainque B, Schwartz K. Familial hypertrophic cardiomyopathy: from mutations to functional defects. Circ Res. 1998;83:580–593. - PubMed
-
- Maron BJ, Bonow RO, Cannon RO, III, Leon MB, Epstein SE. Hypertrophic cardiomyopathy. I. Interrelations of clinical manifestations, pathophysiology, and therapy. N Engl J Med. 1987;316:780–789. - PubMed
-
- Maron BJ, Bonow RO, Cannon RO, III, Leon MB, Epstein SE. Hypertrophic cardiomyopathy. II. Interrelations of clinical manifestations, pathophysiology, and therapy. N Engl J Med. 1987;316:844–852. - PubMed
-
- Marian AJ. Sudden cardiac death in patients with hypertrophic cardiomyopathy: from bench to bedside with an emphasis on genetic markers. Clin Cardiol. 1995;18:189–198. - PubMed
-
- Thierfelder L, et al. α-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere. Cell. 1994;77:701–712. - PubMed

