Distinct roles of prostaglandin H synthases 1 and 2 in T-cell development
- PMID: 10330429
- PMCID: PMC408457
- DOI: 10.1172/JCI6400
Distinct roles of prostaglandin H synthases 1 and 2 in T-cell development
Abstract
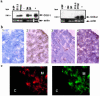
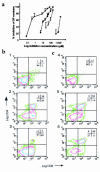
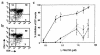
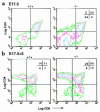
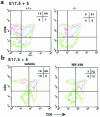
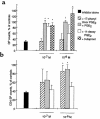
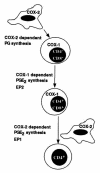
Prostaglandin G and H synthases, or cyclooxygenases (COXs), catalyze the formation of prostaglandins (PGs). Whereas COX-1 is diffusely expressed in lymphoid cells in embryonic day 15.5 thymus, COX-2 expression is sparse, apparently limited to stromal cells. By contrast, COX-2 is predominant in a subset of medullary stromal cells in three- to five-week-old mice. The isozymes also differ in their contributions to lymphocyte development. Thus, experiments with selective COX-1 inhibitors in thymic lobes from normal and recombinase-activating gene-1 knockout mice support a role for this isoform in the transition from CD4(-)CD8(-) double-negative (DN) to CD4(+)CD8(+) double-positive (DP). Concordant data were obtained in COX-1 knockouts. Pharmacological inhibition and genetic deletion of COX-2, by contrast, support its role during early thymocyte proliferation and differentiation and, later, during maturation of the CD4 helper T-cell lineage. PGE2, but not other PGs, can rescue the effects of inhibition of either isoform, although it acts through distinct EP receptor subtypes. COX-dependent PG generation may represent a mechanism of thymic stromal support for T-cell development.
Figures

References
-
- Smith WL, DeWitt DL. Prostaglandin endoperoxide H synthases -1 and -2. Adv Immunol. 1996;62:167–215. - PubMed
-
- Herschman HR, Reddy ST, Xie W. Function and regulation of prostaglandin synthase-2. Adv Exp Med Biol. 1997;407:61–66. - PubMed
-
- Betz M, Fox BS. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol. 1991;146:108–113. - PubMed
-
- Anastassiou ED, Paliogianni F, Balow JP, Yamada H, Boumpas DT. Prostaglandin E2 and other cyclic AMP-elevating agents modulate IL-2 and IL-2R alpha gene expression at multiple levels. J Immunol. 1992;148:2845–2852. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

