A kinetic threshold between negative and positive selection based on the longevity of the T cell receptor-ligand complex
- PMID: 10330432
- PMCID: PMC2193645
- DOI: 10.1084/jem.189.10.1531
A kinetic threshold between negative and positive selection based on the longevity of the T cell receptor-ligand complex
Abstract
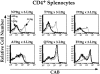
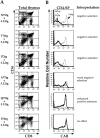
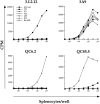
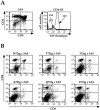
We have developed a unique in vivo system to determine the relationship between endogenous altered peptide ligands and the development of major histocompatibility complex class II- restricted T cells. Our studies use the 3.L2 T cell receptor (TCR) transgenic mouse, in which T cells are specific for Hb(64-76)/I-Ek and positively selected on I-Ek plus self-peptides. To this endogenous peptide repertoire, we have individually added one of six well-characterized 3.L2 ligands. This transgenic approach expands rather than constrains the repertoire of self-peptides. We find that a broad range of ligands produce negative selection of thymocytes in vivo. When compared with the in vitro TCR-ligand binding kinetics, we find that these negatively selecting ligands all have a half-life of 2 s or greater. Additionally, one of two ligands examined with no detectable binding to the 3.L2 TCR and no activity on mature 3.L2 T cells (Q72) enhances the positive selection of transgenic thymocytes in vivo. Together, these data establish a kinetic threshold between negative and positive selection based on the longevity of TCR-ligand complexes.
Figures



References
-
- Kappler JW, Noehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. - PubMed
-
- MacDonald HR, Schneider R, Lees RK, Howe RC, Acha-Orbea H, Festenstein H, Zinkernagel RM, Hengartner H. T-cell receptor Vβ use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature. 1988;332:40–45. - PubMed
-
- Kisielow P, Blüthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+thymocytes. Nature. 1988;333:742–746. - PubMed
-
- Sha WC, Nelson CA, Newberry RD, Kranz DM, Russell JH, Loh DY. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 1988;336:73–79. - PubMed
-
- Bevan MJ. In a radiation chimera host H-2 antigens determine the immune responsiveness of donor cytotoxic cells. Nature. 1977;269:417–419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

