Regenerative potentials evoked in circular smooth muscle of the antral region of guinea-pig stomach
- PMID: 10332102
- PMCID: PMC2269361
- DOI: 10.1111/j.1469-7793.1999.0563t.x
Regenerative potentials evoked in circular smooth muscle of the antral region of guinea-pig stomach
Abstract
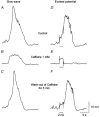
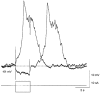
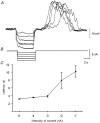
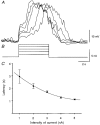
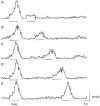
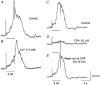
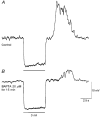
1. Slow waves recorded from the circular smooth muscle layer of guinea-pig antrum consisted of two components, an initial component and a secondary regenerative component. Whereas both components persisted in the presence of nifedipine, the secondary component was abolished by a low concentration of caffeine. 2. Short segments of single bundles of circular muscle were isolated and impaled with two microelectrodes. Depolarizing currents initiated regenerative responses which resembled those initiated during normal slow waves. These responses had partial refractory periods of 20-30 s and were initiated about 1 s after the onset of membrane depolarization. 3. The regenerative responses persisted in the presence of either nifedipine or cobalt ions but were abolished by caffeine, BAPTA or cyclopiazonic acid. 4. The observations suggest that depolarizing membrane potential changes trigger the release of Ca2+ from intracellular stores and this causes a depolarization by activating sets of unidentified ion channels in the membranes of smooth muscle cells of the circular layer of guinea-pig antrum.
Figures



Similar articles
-
Unitary nature of regenerative potentials recorded from circular smooth muscle of guinea-pig antrum.J Physiol. 1999 Aug 15;519 Pt 1(Pt 1):235-50. doi: 10.1111/j.1469-7793.1999.0235o.x. J Physiol. 1999. PMID: 10432354 Free PMC article.
-
Spontaneous electrical activity and associated changes in calcium concentration in guinea-pig gastric smooth muscle.J Physiol. 2002 Apr 1;540(Pt 1):249-60. doi: 10.1113/jphysiol.2001.013306. J Physiol. 2002. PMID: 11927684 Free PMC article.
-
Dual effects of cyclopiazonic acid on excitation of circular smooth muscle isolated from the guinea-pig gastric antrum.J Smooth Muscle Res. 2002 Apr;38(1-2):23-37. doi: 10.1540/jsmr.38.23. J Smooth Muscle Res. 2002. PMID: 12199530
-
Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus.J Physiol. 2000 Apr 1;524 Pt 1(Pt 1):245-65. doi: 10.1111/j.1469-7793.2000.00245.x. J Physiol. 2000. PMID: 10747196 Free PMC article.
-
Factors modifying the frequency of spontaneous activity in gastric muscle.J Physiol. 2006 Nov 1;576(Pt 3):667-74. doi: 10.1113/jphysiol.2006.117093. Epub 2006 Aug 31. J Physiol. 2006. PMID: 16945968 Free PMC article. Review.
Cited by
-
Atypical slow waves generated in gastric corpus provide dominant pacemaker activity in guinea pig stomach.J Physiol. 2005 Dec 1;569(Pt 2):459-65. doi: 10.1113/jphysiol.2005.097907. Epub 2005 Oct 13. J Physiol. 2005. PMID: 16223760 Free PMC article.
-
Pacemaker frequency is increased by sodium nitroprusside in the guinea pig gastric antrum.J Physiol. 2003 Jan 1;546(Pt 1):191-205. doi: 10.1113/jphysiol.2002.027607. J Physiol. 2003. PMID: 12509488 Free PMC article.
-
Effects of imatinib mesylate on spontaneous electrical and mechanical activity in smooth muscle of the guinea-pig stomach.Br J Pharmacol. 2008 May;154(2):451-9. doi: 10.1038/bjp.2008.91. Epub 2008 Apr 14. Br J Pharmacol. 2008. PMID: 18414381 Free PMC article.
-
Distribution of pacemaker function through the tunica muscularis of the canine gastric antrum.J Physiol. 2001 Nov 15;537(Pt 1):237-50. doi: 10.1111/j.1469-7793.2001.0237k.x. J Physiol. 2001. PMID: 11711577 Free PMC article.
-
Interaction between excitatory and inhibitory metabotropic pathways in the guinea-pig antrum.J Physiol. 2003 Jul 1;550(Pt 1):181-9. doi: 10.1113/jphysiol.2003.043273. J Physiol. 2003. PMID: 12879868 Free PMC article.
References
-
- Burns AJ, Herbert TM, Ward SM, Sanders KM. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit immunohistochemistry. Cell and Tissue Research. 1997;290:11–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

