Protection against development of otitis media induced by nontypeable Haemophilus influenzae by both active and passive immunization in a chinchilla model of virus-bacterium superinfection
- PMID: 10338477
- PMCID: PMC96578
- DOI: 10.1128/IAI.67.6.2746-2762.1999
Protection against development of otitis media induced by nontypeable Haemophilus influenzae by both active and passive immunization in a chinchilla model of virus-bacterium superinfection
Erratum in
- Infect Immun 1999 Oct;67(10):5545
Abstract
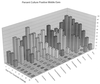
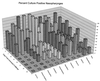
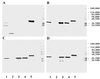
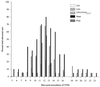
Three separate studies, two involving active-immunization regimens and one involving a passive-transfer protocol, were conducted to initially screen and ultimately more fully assess several nontypeable Haemophilus influenzae outer membrane proteins or their derivatives for their relative protective efficacy in chinchilla models of otitis media. Initial screening of these antigens (P5-fimbrin, lipoprotein D, and P6), delivered singly or in combination with either Freund's adjuvant or alum, indicated that augmented bacterial clearance from the nasopharynx, the middle ears, or both anatomical sites could be induced by parenteral immunization with P5-fimbrin combined with lipoprotein D, lipoprotein D alone, or the synthetic chimeric peptide LB1 (derived from P5-fimbrin), respectively. Data from a second study, wherein chinchillas were immunized with LB1 or lipoprotein D, each delivered with alum, again indicated that clearance of nontypeable H. influenzae could be augmented by immunization with either of these immunogens; however, when this adjuvant was used, both antibody titers in serum and efficacy were reduced. A third study was performed to investigate passive delivery of antisera directed against either LB1, lipoprotein D, nonacylated lipoprotein D, or a unique recombinant peptide designated LPD-LB1(f)2,1,3. The last three antiserum pools were generated by using the combined adjuvant of alum plus monophosphoryl lipid A. Passive transfer of sera specific for LB1 or LPD-LB1(f)2,1,3 to adenovirus-compromised chinchillas, prior to intranasal challenge with nontypeable H. influenzae, significantly reduced the severity of signs and incidence of otitis media which developed (P </= 0.001). Collectively, these data indicate the continued merit of further developing LB1 and LPD-LB1(f)2,1,3 as components of vaccines for otitis media.
Figures




References
-
- Akkoyunlu M, Forsgren A. Local and systemic antibody levels against protein D of Haemophilus influenzae following immunization and infection in rats. APMIS. 1996;104:709–717. - PubMed
-
- Akkoyunlu M, Melhus A, Capiau C, Van Opstal O, Forsgren A. The acylated form of protein D of Haemophilus influenzae is more immunogenic than the nonacylated form and elicits an adjuvant effect when it is used as a carrier conjugated to polyribosyl ribitol phosphate. Infect Immun. 1997;65:5010–5016. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

