Platelet-activating factor induces nitric oxide synthesis in Trypanosoma cruzi-infected macrophages and mediates resistance to parasite infection in mice
- PMID: 10338485
- PMCID: PMC96586
- DOI: 10.1128/IAI.67.6.2810-2814.1999
Platelet-activating factor induces nitric oxide synthesis in Trypanosoma cruzi-infected macrophages and mediates resistance to parasite infection in mice
Abstract
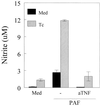
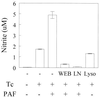
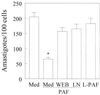
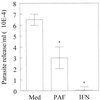
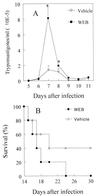
Trypanosoma cruzi replicates in nucleated cells and is susceptible to being killed by gamma interferon-activated macrophages through a mechanism dependent upon NO biosynthesis. In the present study, the role of platelet-activating factor (PAF) in the induction of NO synthesis and in the activation of the trypanocidal activity of macrophages was investigated. In vitro, PAF induced NO secretion by T. cruzi-infected macrophages and the secreted NO inhibited intracellular parasite growth. The addition of a PAF antagonist, WEB 2170, inhibited both NO biosynthesis and trypanocidal activity. The inducible NO synthase/L-arginine pathway mediated trypanocidal activity, since it was inhibited by treatment with L-N-monomethyl arginine (L-NMMA), an L-arginine analog. PAF-mediated NO production in infected macrophages appears to be dependent on tumor necrosis alpha (TNF-alpha) production, since the addition of a neutralizing anti-TNF-alpha monoclonal antibody mAb inhibited NO synthesis. To test the role of PAF in mediating resistance or susceptibility to T. cruzi infection, infected mice were treated with WEB 2170, a PAF antagonist. These animals had higher parasitemia and earlier mortality than did vehicle-treated mice. Taken together, our results suggest that PAF belongs to a group of mediators that coordinate the mechanisms of resistance to infections with intracellular parasites.
Figures


References
-
- Bussolati B, Mariano F, Cignetti A, Guarini A, Cambi V, Foa R, Piccoli G, Camussi G. Platelet-activating factor synthesized by IL-12-stimulated polymorphonuclear neutrophils and NK cells mediates chemotaxis. J Immunol. 1998;161:1493–1500. - PubMed
-
- Braquet P, Touqui L, Shen T Y, Vargaftig B B. Perspectives in platelet-activating factor research. Pharmacol Rev. 1987;39:97–145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

