Requirement of MrpH for mannose-resistant Proteus-like fimbria-mediated hemagglutination by Proteus mirabilis
- PMID: 10338487
- PMCID: PMC96588
- DOI: 10.1128/IAI.67.6.2822-2833.1999
Requirement of MrpH for mannose-resistant Proteus-like fimbria-mediated hemagglutination by Proteus mirabilis
Abstract
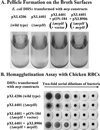
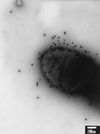
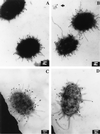
Two new genes, mrpH and mrpJ, were identified downstream of mrpG in the mrp gene cluster encoding mannose-resistant Proteus-like (MR/P) fimbriae of uropathogenic Proteus mirabilis. Since the predicted MrpH has 30% amino acid sequence identity to PapG, the Galalpha(1-4)Gal-binding adhesin of Escherichia coli P fimbriae, we hypothesized that mrpH encodes the functional MR/P hemagglutinin. MR/P fimbriae, expressed in E. coli DH5alpha, conferred on bacteria both the ability to cause mannose-resistant hemagglutination and the ability to aggregate to form pellicles on the broth surface. Both a DeltamrpH mutant expressed in E. coli DH5alpha and an isogenic mrpH::aphA mutant of P. mirabilis were unable to produce normal MR/P fimbriae efficiently, suggesting that MrpH was involved in fimbrial assembly. Amino acid residue substitution of the N-terminal cysteine residues (C66S and C128S) of MrpH abolished the receptor-binding activity (hemagglutinating ability) of MrpH but allowed normal fimbrial assembly, supporting the notion that MrpH was the functional MR/P hemagglutinin. Immunogold electron microscopy of P. mirabilis HI4320 revealed that MrpH was located at the tip of MR/P fimbriae, also consistent with its role in receptor binding. The isogenic mrpH::aphA mutant of HI4320 was less able to colonize the urine, bladder, and kidneys in a mouse model of ascending urinary tract infection (P < 0.01), and therefore MR/P fimbriae contribute significantly to bacterial colonization in mice. While there are similarities between P. mirabilis MR/P and E. coli P fimbriae, there are more notable differences: (i) synthesis of the MrpH adhesin is required to initiate fimbrial assembly, (ii) MR/P fimbriae confer an aggregation phenotype, (iii) site-directed mutation of specific residues can abolish receptor binding but allows fimbrial assembly, and (iv) mutation of the adhesin gene abolishes virulence in a mouse model of ascending urinary tract infection.
Figures







Similar articles
-
Pathogenesis of Proteus mirabilis Infection.EcoSal Plus. 2018 Feb;8(1):10.1128/ecosalplus.ESP-0009-2017. doi: 10.1128/ecosalplus.ESP-0009-2017. EcoSal Plus. 2018. PMID: 29424333 Free PMC article. Review.
-
Proteus mirabilis mannose-resistant, Proteus-like fimbriae: MrpG is located at the fimbrial tip and is required for fimbrial assembly.Infect Immun. 1997 Apr;65(4):1327-34. doi: 10.1128/iai.65.4.1327-1334.1997. Infect Immun. 1997. PMID: 9119470 Free PMC article.
-
In vivo phase variation of MR/P fimbrial gene expression in Proteus mirabilis infecting the urinary tract.Mol Microbiol. 1997 Mar;23(5):1009-19. doi: 10.1046/j.1365-2958.1997.2791645.x. Mol Microbiol. 1997. PMID: 9076737
-
Proteus mirabilis MR/P fimbriae: molecular cloning, expression, and nucleotide sequence of the major fimbrial subunit gene.J Bacteriol. 1993 Jan;175(2):457-64. doi: 10.1128/jb.175.2.457-464.1993. J Bacteriol. 1993. PMID: 8093447 Free PMC article.
-
Fimbriae of uropathogenic Proteus mirabilis.FEMS Immunol Med Microbiol. 2007 Oct;51(1):1-7. doi: 10.1111/j.1574-695X.2007.00284.x. Epub 2007 Jul 19. FEMS Immunol Med Microbiol. 2007. PMID: 17640292 Review.
Cited by
-
Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis.Clin Microbiol Rev. 2008 Jan;21(1):26-59. doi: 10.1128/CMR.00019-07. Clin Microbiol Rev. 2008. PMID: 18202436 Free PMC article. Review.
-
Pathogenesis of Proteus mirabilis Infection.EcoSal Plus. 2018 Feb;8(1):10.1128/ecosalplus.ESP-0009-2017. doi: 10.1128/ecosalplus.ESP-0009-2017. EcoSal Plus. 2018. PMID: 29424333 Free PMC article. Review.
-
Structure, Function, and Assembly of Adhesive Organelles by Uropathogenic Bacteria.Microbiol Spectr. 2015 Oct;3(5):10.1128/microbiolspec.UTI-0018-2013. doi: 10.1128/microbiolspec.UTI-0018-2013. Microbiol Spectr. 2015. PMID: 26542038 Free PMC article. Review.
-
Proteus mirabilis fimbriae- and urease-dependent clusters assemble in an extracellular niche to initiate bladder stone formation.Proc Natl Acad Sci U S A. 2016 Apr 19;113(16):4494-9. doi: 10.1073/pnas.1601720113. Epub 2016 Apr 4. Proc Natl Acad Sci U S A. 2016. PMID: 27044107 Free PMC article.
-
From Catheter to Kidney Stone: The Uropathogenic Lifestyle of Proteus mirabilis.Trends Microbiol. 2017 Apr;25(4):304-315. doi: 10.1016/j.tim.2016.11.015. Epub 2016 Dec 22. Trends Microbiol. 2017. PMID: 28017513 Free PMC article. Review.
References
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 2. New York, N.Y: John Wiley & Sons, Inc.; 1995. pp. 10.8.1–10.8.17.
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 2. New York, N.Y: John Wiley & Sons, Inc.; 1995. pp. 16.6.1–16.6.14.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

