Access of antibody or trypsin to an integral outer membrane protein (P66) of Borrelia burgdorferi is hindered by Osp lipoproteins
- PMID: 10338494
- PMCID: PMC96595
- DOI: 10.1128/IAI.67.6.2874-2883.1999
Access of antibody or trypsin to an integral outer membrane protein (P66) of Borrelia burgdorferi is hindered by Osp lipoproteins
Abstract
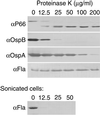
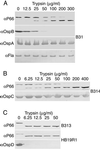
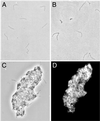
The outer membrane of Borrelia burgdorferi, the Lyme disease agent, contains lipoproteins anchored by their lipid moieties and integral proteins with membrane-spanning regions. We used the techniques of in situ proteolysis, immunofluorescence, in vitro growth inhibition, and cross-linking with formaldehyde to characterize topological relationships between P66, an integral membrane protein, and selected Osp lipoproteins of B. burgdorferi. Protease treatment of intact spirochetes cleaved P66 and Osp proteins but not the periplasmic flagellin or the BmpA protein of the cytoplasmic membrane. P66 of cells lacking OspA, OspB, and OspC was more susceptible to trypsin cleavage than was P66 of cells with these Osp proteins. A monoclonal antibody against the surface loop of P66 bound, agglutinated, and inhibited the growth of viable spirochetes lacking OspA, OspB, OspC, and OspD but not of the cells that expressed OspA, OspC, and/or OspD. When cells were fixed, the antibody bound to cells that express OspD and OspC but still not to cells with OspA. The close association of OspA and P66 was confirmed by the crosslinking of the two proteins by formaldehyde. These results show that Osp proteins, particularly OspA, limit the access of antibody or trypsin to the surface loop region of P66. The proximity and possible contact between P66 and OspA (or other Osp proteins) may hinder the effectiveness of antibodies to what otherwise would be an appropriate vaccine target.
Figures




References
-
- Anonymous. Recomendations for test performance and interpretation from the Second International Conference on Serologic Diagnosis of Lyme Disease. Morbid Mortal Weekly Rep. 1995;44:590–591. - PubMed
-
- Åsbrink E, Hovmark A, Hederstedt B. The spirochetal etiology of acrodermatitis chronica atrophicans Herxheimer. Acta Dermatol Venereol. 1984;64:506–512. - PubMed
-
- Barbour A G, Garon C F. Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science. 1987;237:409–411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases


