Molecular characterization and human T-cell responses to a member of a novel Mycobacterium tuberculosis mtb39 gene family
- PMID: 10338503
- PMCID: PMC96604
- DOI: 10.1128/IAI.67.6.2941-2950.1999
Molecular characterization and human T-cell responses to a member of a novel Mycobacterium tuberculosis mtb39 gene family
Abstract
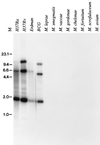
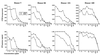
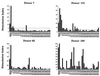
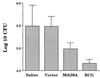
We have used expression screening of a genomic Mycobacterium tuberculosis library with tuberculosis (TB) patient sera to identify novel genes that may be used diagnostically or in the development of a TB vaccine. Using this strategy, we have cloned a novel gene, termed mtb39a, that encodes a 39-kDa protein. Molecular characterization revealed that mtb39a is a member of a family of three highly related genes that are conserved among strains of M. tuberculosis and Mycobacterium bovis BCG but not in other mycobacterial species tested. Immunoblot analysis demonstrated the presence of Mtb39A in M. tuberculosis lysate but not in culture filtrate proteins (CFP), indicating that it is not a secreted antigen. This conclusion is strengthened by the observation that a human T-cell clone specific for purified recombinant Mtb39A protein recognized autologous dendritic cells infected with TB or pulsed with purified protein derivative (PPD) but did not respond to M. tuberculosis CFP. Purified recombinant Mtb39A elicited strong T-cell proliferative and gamma interferon responses in peripheral blood mononuclear cells from 9 of 12 PPD-positive individuals tested, and overlapping peptides were used to identify a minimum of 10 distinct T-cell epitopes. Additionally, mice immunized with mtb39a DNA have shown increased protection from M. tuberculosis challenge, as indicated by a reduction of bacterial load. The human T-cell responses and initial animal studies provide support for further evaluation of this antigen as a possible component of a subunit vaccine for M.tuberculosis.
Figures
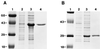




Similar articles
-
Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG.J Clin Microbiol. 2000 Sep;38(9):3285-90. doi: 10.1128/JCM.38.9.3285-3290.2000. J Clin Microbiol. 2000. PMID: 10970372 Free PMC article.
-
Evaluation of T-cell responses to novel RD1- and RD2-encoded Mycobacterium tuberculosis gene products for specific detection of human tuberculosis infection.Infect Immun. 2004 May;72(5):2574-81. doi: 10.1128/IAI.72.5.2574-2581.2004. Infect Immun. 2004. PMID: 15102765 Free PMC article.
-
PPE protein (Rv3873) from DNA segment RD1 of Mycobacterium tuberculosis: strong recognition of both specific T-cell epitopes and epitopes conserved within the PPE family.Infect Immun. 2003 Nov;71(11):6116-23. doi: 10.1128/IAI.71.11.6116-6123.2003. Infect Immun. 2003. PMID: 14573626 Free PMC article.
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
-
Development of new vaccines and diagnostic reagents against tuberculosis.Mol Immunol. 2002 Sep;39(1-2):113-9. doi: 10.1016/s0161-5890(02)00048-2. Mol Immunol. 2002. PMID: 12213334 Review.
Cited by
-
Induction and regulation of T-cell immunity by the novel tuberculosis vaccine M72/AS01 in South African adults.Am J Respir Crit Care Med. 2013 Aug 15;188(4):492-502. doi: 10.1164/rccm.201208-1385OC. Am J Respir Crit Care Med. 2013. PMID: 23306546 Free PMC article. Clinical Trial.
-
Identification by mass spectrometry of CD8(+)-T-cell Mycobacterium tuberculosis epitopes within the Rv0341 gene product.Infect Immun. 2002 Jun;70(6):2926-32. doi: 10.1128/IAI.70.6.2926-2932.2002. Infect Immun. 2002. PMID: 12010981 Free PMC article.
-
Translational mini-review series on vaccines: Development and evaluation of improved vaccines against tuberculosis.Clin Exp Immunol. 2007 Mar;147(3):401-11. doi: 10.1111/j.1365-2249.2006.03306.x. Clin Exp Immunol. 2007. PMID: 17302888 Free PMC article. Review.
-
Endogenous human cytomegalovirus gB is presented efficiently by MHC class II molecules to CD4+ CTL.J Exp Med. 2005 Oct 17;202(8):1109-19. doi: 10.1084/jem.20050162. Epub 2005 Oct 10. J Exp Med. 2005. PMID: 16216889 Free PMC article.
-
Tuberculosis vaccine development: from classic to clinical candidates.Eur J Clin Microbiol Infect Dis. 2020 Aug;39(8):1405-1425. doi: 10.1007/s10096-020-03843-6. Epub 2020 Feb 15. Eur J Clin Microbiol Infect Dis. 2020. PMID: 32060754 Free PMC article. Review.
References
-
- Andersen P, Andersen A B, Sorensen A L, Nagai S. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J Immunol. 1995;154:3359–3372. - PubMed
-
- Arachi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991;72:1–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

