Proteasome activity is required for anthrax lethal toxin to kill macrophages
- PMID: 10338520
- PMCID: PMC96621
- DOI: 10.1128/IAI.67.6.3055-3060.1999
Proteasome activity is required for anthrax lethal toxin to kill macrophages
Abstract
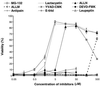
Anthrax lethal toxin (LeTx), consisting of protective antigen (PA) and lethal factor (LF), rapidly kills primary mouse macrophages and macrophage-like cell lines such as RAW 264.7. LF is translocated by PA into the cytosol of target cells, where it acts as a metalloprotease to cleave mitogen-activated protein kinase kinase 1 (MEK1) and possibly other proteins. In this study, we show that proteasome inhibitors such as acetyl-Leu-Leu-norleucinal, MG132, and lactacystin efficiently block LeTx cytotoxicity, whereas other protease inhibitors do not. The inhibitor concentrations that block LF cytotoxicity are similar to those that inhibit the proteasome-dependent IkappaB-alpha degradation induced by lipopolysaccharide. The inhibitors did not interfere with the proteolytic cleavage of MEK1 in LeTx-treated cells, indicating that they do not directly block the proteolytic activity of LF. However, the proteasome inhibitors did prevent ATP depletion, an early effect of LeTx. No overall activation of the proteasome by LeTx was detected, as shown by the cleavage of fluorogenic substrates of the proteasome. All of these results suggest that the proteasome mediates a toxic process initiated by LF in the cell cytosol. This process probably involves degradation of unidentified molecules that are essential for macrophage homeostasis. Moreover, this proteasome-dependent process is an early step in LeTx intoxication, but it is downstream of the cleavage by LF of MEK1 or other putative substrates.
Figures
Similar articles
-
Critical role of the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase-3 signaling pathway in recovery from anthrax lethal toxin-induced cell cycle arrest and MEK cleavage in macrophages.J Biol Chem. 2007 Dec 14;282(50):36230-9. doi: 10.1074/jbc.M707622200. Epub 2007 Oct 19. J Biol Chem. 2007. PMID: 17951252
-
Anthrax lethal factor cleaves MKK3 in macrophages and inhibits the LPS/IFNgamma-induced release of NO and TNFalpha.FEBS Lett. 1999 Nov 26;462(1-2):199-204. doi: 10.1016/s0014-5793(99)01502-1. FEBS Lett. 1999. PMID: 10580119
-
Toxin-induced resistance in Bacillus anthracis lethal toxin-treated macrophages.Proc Natl Acad Sci U S A. 2003 Oct 14;100(21):12426-31. doi: 10.1073/pnas.2134042100. Epub 2003 Sep 30. Proc Natl Acad Sci U S A. 2003. PMID: 14519843 Free PMC article.
-
Consequences and utility of the zinc-dependent metalloprotease activity of anthrax lethal toxin.Toxins (Basel). 2010 May;2(5):1038-53. doi: 10.3390/toxins2051038. Epub 2010 May 11. Toxins (Basel). 2010. PMID: 22069624 Free PMC article. Review.
-
Anthrax toxin: a tripartite lethal combination.FEBS Lett. 2002 Nov 20;531(3):384-8. doi: 10.1016/s0014-5793(02)03609-8. FEBS Lett. 2002. PMID: 12435580 Review.
Cited by
-
Binary bacterial toxins: biochemistry, biology, and applications of common Clostridium and Bacillus proteins.Microbiol Mol Biol Rev. 2004 Sep;68(3):373-402, table of contents. doi: 10.1128/MMBR.68.3.373-402.2004. Microbiol Mol Biol Rev. 2004. PMID: 15353562 Free PMC article. Review.
-
Proteolysis, synaptic plasticity and memory.Neurobiol Learn Mem. 2017 Feb;138:98-110. doi: 10.1016/j.nlm.2016.09.003. Epub 2016 Sep 7. Neurobiol Learn Mem. 2017. PMID: 27614141 Free PMC article. Review.
-
The N-end rule ubiquitin ligase UBR2 mediates NLRP1B inflammasome activation by anthrax lethal toxin.EMBO J. 2019 Jul 1;38(13):e101996. doi: 10.15252/embj.2019101996. Epub 2019 May 6. EMBO J. 2019. PMID: 31268597 Free PMC article.
-
Reduced expression of CD45 protein-tyrosine phosphatase provides protection against anthrax pathogenesis.J Biol Chem. 2009 May 8;284(19):12874-85. doi: 10.1074/jbc.M809633200. Epub 2009 Mar 6. J Biol Chem. 2009. PMID: 19269962 Free PMC article.
-
Activation of the Nlrp1b inflammasome by reduction of cytosolic ATP.Infect Immun. 2013 Feb;81(2):570-9. doi: 10.1128/IAI.01003-12. Epub 2012 Dec 10. Infect Immun. 2013. PMID: 23230290 Free PMC article.
References
-
- Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. - PubMed
-
- Craiu A, Gaczynska M, Akopian T, Gramm C F, Fenteany G, Goldberg A L, Rock K L. Lactacystin and clasto-lactacystin beta-lactone modify multiple proteasome beta-subunits and inhibit intracellular protein degradation and major histocompatibility complex class I antigen presentation. J Biol Chem. 1997;272:13437–13445. - PubMed
-
- DeMartino G N, McCullough M L, Reckelhoff J F, Croall D E, Ciechanover A, McGuire M J. ATP-stimulated degradation of endogenous proteins in cell-free extracts of BHK 21/C13 fibroblasts. A key role for the proteinase, macropain, in the ubiquitin-dependent degradation of short-lived proteins. Biochim Biophys Acta. 1991;1073:299–308. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

