A role for the light-dependent phosphorylation of visual arrestin
- PMID: 10339543
- PMCID: PMC26837
- DOI: 10.1073/pnas.96.11.6072
A role for the light-dependent phosphorylation of visual arrestin
Abstract
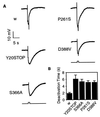
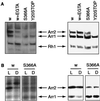
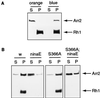
Arrestins are regulatory proteins that participate in the termination of G protein-mediated signal transduction. The major arrestin in the Drosophila visual system, Arrestin 2 (Arr2), is phosphorylated in a light-dependent manner by a Ca2+/calmodulin-dependent protein kinase and has been shown to be essential for the termination of the visual signaling cascade in vivo. Here, we report the isolation of nine alleles of the Drosophila photoreceptor cell-specific arr2 gene. Flies carrying each of these alleles underwent light-dependent retinal degeneration and displayed electrophysiological defects typical of previously identified arrestin mutants, including an allele encoding a protein that lacks the major Ca2+/calmodulin-dependent protein kinase site. The phosphorylation mutant had very low levels of phosphorylation and lacked the light-dependent phosphorylation observed with wild-type Arr2. Interestingly, we found that the Arr2 phosphorylation mutant was still capable of binding to rhodopsin; however, it was unable to release from membranes once rhodopsin had converted back to its inactive form. This finding suggests that phosphorylation of arrestin is necessary for the release of arrestin from rhodopsin. We propose that the sequestering of arrestin to membranes is a possible mechanism for retinal disease associated with previously identified rhodopsin alleles in humans.
Figures
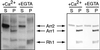

References
-
- Baldwin J M. Curr Opin Cell Biol. 1994;6:180–190. - PubMed
-
- Ji T H, Grossmann M, Ji I. J Biol Chem. 1998;273:17299–17302. - PubMed
-
- Ferguson S S, Barak L S, Zhang J, Caron M G. Can J Physiol Pharmacol. 1996;74:1095–1110. - PubMed
-
- Palczewski K, Saari J C. Curr Opin Neurobiol. 1997;7:500–504. - PubMed
-
- Carman C, Benovic J. Curr Opin Neurobiol. 1998;8:335–344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

