Mutation R120G in alphaB-crystallin, which is linked to a desmin-related myopathy, results in an irregular structure and defective chaperone-like function
- PMID: 10339554
- PMCID: PMC26848
- DOI: 10.1073/pnas.96.11.6137
Mutation R120G in alphaB-crystallin, which is linked to a desmin-related myopathy, results in an irregular structure and defective chaperone-like function
Abstract
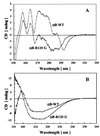
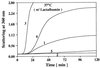
alphaB-crystallin, a member of the small heat shock protein family, possesses chaperone-like function. Recently, it has been shown that a missense mutation in alphaB-crystallin, R120G, is genetically linked to a desmin-related myopathy as well as to cataracts [Vicart, P., Caron, A., Guicheney, P., Li, A., Prevost, M.-C., Faure, A., Chateau, D., Chapon, F., Tome, F., Dupret, J.-M., et al. (1998) Nat. Genet. 20, 92-95]. By using alpha-lactalbumin, alcohol dehydrogenase, and insulin as target proteins, in vitro assays indicated that R120G alphaB-crystallin had reduced or completely lost chaperone-like function. The addition of R120G alphaB-crystallin to unfolding alpha-lactalbumin enhanced the kinetics and extent of its aggregation. R120G alphaB-crystallin became entangled with unfolding alpha-lactalbumin and was a major portion of the resulting insoluble pellet. Similarly, incubation of R120G alphaB-crystallin with alcohol dehydrogenase and insulin also resulted in the presence of R120G alphaB-crystallin in the insoluble pellets. Far and near UV CD indicate that R120G alphaB-crystallin has decreased beta-sheet secondary structure and an altered aromatic residue environment compared with wild-type alphaB-crystallin. The apparent molecular mass of R120G alphaB-crystallin, as determined by gel filtration chromatography, is 1.4 MDa, which is more than twice the molecular mass of wild-type alphaB-crystallin (650 kDa). Images obtained from cryoelectron microscopy indicate that R120G alphaB-crystallin possesses an irregular quaternary structure with an absence of a clear central cavity. The results of this study show, through biochemical analysis, that an altered structure and defective chaperone-like function of alphaB-crystallin are associated with a point mutation that leads to a desmin-related myopathy and cataracts.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

