Shedding light on the dark and weakly fluorescent states of green fluorescent proteins
- PMID: 10339561
- PMCID: PMC26855
- DOI: 10.1073/pnas.96.11.6177
Shedding light on the dark and weakly fluorescent states of green fluorescent proteins
Abstract
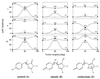
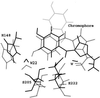
Recent experiments on various similar green fluorescent protein (GFP) mutants at the single-molecule level and in solution provide evidence of previously unknown short- and long-lived "dark" states and of related excited-state decay channels. Here, we present quantum chemical calculations on cis-trans photoisomerization paths of neutral, anionic, and zwitterionic GFP chromophores in their ground and first singlet excited states that explain the observed behaviors from a common perspective. The results suggest that favorable radiationless decay channels can exist for the different protonation states along these isomerizations, which apparently proceed via conical intersections. These channels are suggested to rationalize the observed dramatic reduction of fluorescence in solution. The observed single-molecule fast blinking is attributed to conversions between the fluorescent anionic and the dark zwitterionic forms whereas slow switching is attributed to conversions between the anionic and the neutral forms. The predicted nonadiabatic crossings are seen to rationalize the origins of a variety of experimental observations on a common basis and may have broad implications for photobiophysical mechanisms in GFP.
Figures
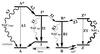
References
-
- Tsien R Y. Annu Rev Biochem. 1998;67:509–544. - PubMed
-
- Gai F, Hasson K C, McDonald J C, Anfinrud P A. Science. 1998;279:1886–1891. - PubMed
-
- Genick U K, Soltis S M, Kuhn P, Canestrelli I L, Getzoff E D. Nature (London) 1998;392:206–209. - PubMed
-
- Genick U K, Borgstahl G E, Ng K, Ren Z, Pradervand C, Burke P M, Srajer V, Teng T Y, Schildkamp W, McRee D E, et al. Science. 1997;275:1471–1475. - PubMed
-
- Perman B, Srajer V, Ren Z, Teng T, Pradervand C, Ursby T, Bourgeois D, Schotte F, Wulff M, Kort R, et al. Science. 1998;279:1946–1950. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

