Receptors for oxidized low-density lipoprotein on elicited mouse peritoneal macrophages can recognize both the modified lipid moieties and the modified protein moieties: implications with respect to macrophage recognition of apoptotic cells
- PMID: 10339590
- PMCID: PMC26884
- DOI: 10.1073/pnas.96.11.6347
Receptors for oxidized low-density lipoprotein on elicited mouse peritoneal macrophages can recognize both the modified lipid moieties and the modified protein moieties: implications with respect to macrophage recognition of apoptotic cells
Abstract
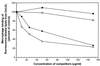
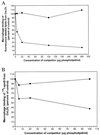
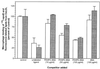
It has been shown previously that the binding of oxidized low-density lipoprotein (OxLDL) to resident mouse peritoneal macrophages can be inhibited (up to 70%) by the apoprotein B (apoB) isolated from OxLDL, suggesting that macrophage recognition of OxLDL is primarily dependent on its modified protein moiety. However, recent experiments have demonstrated that the lipids isolated from OxLDL and reconstituted into a microemulsion can also strongly inhibit uptake of OxLDL (up to 80%). The present studies show that lipid microemulsions prepared from OxLDL bind to thioglycollate-elicited macrophages at 4 degrees C in a saturable fashion and inhibit the binding of intact OxLDL and also of the apoB from OxLDL. Reciprocally, the binding of the OxLDL-lipid microemulsions was strongly inhibited by intact OxLDL. A conjugate of synthetic 1-palmitoyl 2(5-oxovaleroyl) phosphatidylcholine (an oxidation product of 1-palmitoyl 2-arachidonoyl phosphatidylcholine) with serum albumin, shown previously to inhibit macrophage binding of intact OxLDL, also inhibited the binding of both the apoprotein and the lipid microemulsions prepared from OxLDL. Finally, a monoclonal antibody against oxidized phospholipids, one that inhibits binding of intact OxLDL to macrophages, also inhibited the binding of both the resolubilized apoB and the lipid microemulsions prepared from OxLDL. These studies support the conclusions that: (i) at least some of the macrophage receptors for oxidized LDL can recognize both the lipid and the protein moieties; and (ii) oxidized phospholipids, in the lipid phase of the lipoprotein and/or covalently linked to the apoB of OxLDL, likely play a role in that recognition.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

