Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition
- PMID: 10339591
- PMCID: PMC26885
- DOI: 10.1073/pnas.96.11.6353
Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition
Abstract
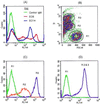
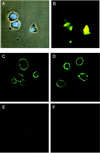
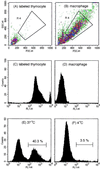
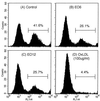
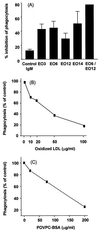
Apoptosis is recognized as important for normal cellular homeostasis in multicellular organisms. Although there have been great advances in our knowledge of the molecular events regulating apoptosis, much less is known about the receptors on phagocytes responsible for apoptotic cell recognition and phagocytosis or the ligands on apoptotic cells mediating such recognition. The observations that apoptotic cells are under increased oxidative stress and that oxidized low-density lipoprotein (OxLDL) competes with apoptotic cells for macrophage binding suggested the hypothesis that both OxLDL and apoptotic cells share oxidatively modified moieties on their surfaces that serve as ligands for macrophage recognition. To test this hypothesis, we used murine monoclonal autoantibodies that bind to oxidation-specific epitopes on OxLDL. In particular, antibodies EO6 and EO3 recognize oxidized phospholipids, including 1-palmitoyl 2-(5-oxovaleroyl) phosphatidylcholine (POVPC), and antibodies EO12 and EO14 recognize malondialdehyde-lysine, as in malondialdehyde-LDL. Using FACS analysis, we demonstrated that each of these EO antibodies bound to apoptotic cells but not to normal cells, whereas control IgM antibodies did not. Confocal microscopy demonstrated cell-surface expression of the oxidation-specific epitopes on apoptotic cells. Furthermore, each of these antibodies inhibited the phagocytosis of apoptotic cells by elicited peritoneal macrophages, as did OxLDL. In addition, an adduct of POVPC with BSA also effectively prevented phagocytosis. These data demonstrate that apoptotic cells express oxidation-specific epitopes-including oxidized phospholipids-on their cell surface, and that these serve as ligands for recognition and phagocytosis by elicited macrophages.
Figures

References
-
- Cohen J J. Immunol Today. 1993;14:126–130. - PubMed
-
- Dixit V M, Green D R, Reed J C, Thornberry N A, Lazebnik Y, Evan G, Littlewood T, Adams J M, Cory S. Science. 1998;281:1305–1326.
-
- Savill J. Brit Med Bull. 1997;53:491–508. - PubMed
-
- Fadok V A, Voelker D R, Campbell P A, Cohen J J, Bratton D L, Henson P M. J Immunol. 1992;148:2207–2216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

