Pressure-mediated oligonucleotide transfection of rat and human cardiovascular tissues
- PMID: 10339601
- PMCID: PMC26895
- DOI: 10.1073/pnas.96.11.6411
Pressure-mediated oligonucleotide transfection of rat and human cardiovascular tissues
Abstract
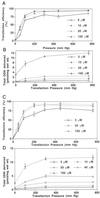
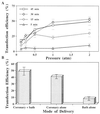
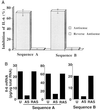
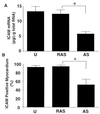
The application of gene therapy to human disease is currently restricted by the relatively low efficiency and potential hazards of methods of oligonucleotide or gene delivery. Antisense or transcription factor decoy oligonucleotides have been shown to be effective at altering gene expression in cell culture expreriments, but their in vivo application is limited by the efficiency of cellular delivery, the intracellular stability of the compounds, and their duration of activity. We report herein the development of a highly efficient method for naked oligodeoxynucleotide (ODN) transfection into cardiovascular tissues by using controlled, nondistending pressure without the use of viral vectors, lipid formulations, or exposure to other adjunctive, potentially hazardous substances. In this study, we have documented the ability of ex vivo, pressure-mediated transfection to achieve nuclear localization of fluorescent (FITC)-labeled ODN in approximately 90% and 50% of cells in intact human saphenous vein and rat myocardium, respectively. We have further documented that pressure-mediated delivery of antisense ODN can functionally inhibit target gene expression in both of these tissues in a sequence-specific manner at the mRNA and protein levels. This oligonucleotide transfection system may represent a safe means of achieving the intraoperative genetic engineering of failure-resistant human bypass grafts and may provide an avenue for the genetic manipultation of cardiac allograft rejection, allograft vasculopathy, or other transplant diseases.
Figures


References
-
- Simons M, Edelman E R, DeKeyser J L, Langer R, Rosenberg R D. Nature (London) 1992;359:67–70. - PubMed
-
- Ohno T, Gordon D, San H, Pompili V J, Imperiale M J, Nabel G J, Nabel E G. Science. 1994;265:781–784. - PubMed
-
- Chang M W, Barr E, Seltzer J, Jiang Y Q, Nabel G J, Nabel E G, Parmacek M S, Leiden J M. Science. 1995;26:518–522. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

