Dual pathways for regulation of root branching by nitrate
- PMID: 10339622
- PMCID: PMC26916
- DOI: 10.1073/pnas.96.11.6529
Dual pathways for regulation of root branching by nitrate
Abstract
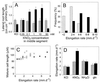
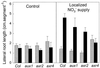
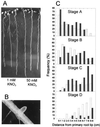
Root development is extremely sensitive to variations in nutrient supply, but the mechanisms are poorly understood. We have investigated the processes by which nitrate (NO3-), depending on its availability and distribution, can have both positive and negative effects on the development and growth of lateral roots. When Arabidopsis roots were exposed to a locally concentrated supply of NO3- there was no increase in lateral root numbers within the NO3--rich zone, but there was a localized 2-fold increase in the mean rate of lateral root elongation, which was attributable to a corresponding increase in the rate of cell production in the lateral root meristem. Localized applications of other N sources did not stimulate lateral root elongation, consistent with previous evidence that the NO3- ion is acting as a signal rather than a nutrient. The axr4 auxin-resistant mutant was insensitive to the stimulatory effect of NO3-, suggesting an overlap between the NO3- and auxin response pathways. High rates of NO3- supply to the roots had a systemic inhibitory effect on lateral root development that acted specifically at the stage when the laterals had just emerged from the primary root, apparently delaying final activation of the lateral root meristem. A nitrate reductase-deficient mutant showed increased sensitivity to this systemic inhibitory effect, suggesting that tissue NO3- levels may play a role in generating the inhibitory signal. We present a model in which root branching is modulated by opposing signals from the plant's internal N status and the external supply of NO3-.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

