Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway
- PMID: 10339626
- PMCID: PMC26920
- DOI: 10.1073/pnas.96.11.6553
Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway
Abstract
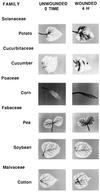
Hydrogen peroxide (H2O2) generated in response to wounding can be detected at wound sites and in distal leaf veins within 1 hr after wounding. The response is systemic and maximizes at about 4-6 hr in both wounded and unwounded leaves, and then declines. The timing of the response corresponds with an increase in wound-inducible polygalacturonase (PG) mRNA and enzyme activity previously reported, suggesting that oligogalacturonic acid (OGA) fragments produced by PG are triggering the H2O2 response. Systemin, OGA, chitosan, and methyl jasmonate (MJ) all induce the accumulation of H2O2 in leaves. Tomato plants transformed with an antisense prosystemin gene produce neither PG activity or H2O2 in leaves in response to wounding, implicating systemin as a primary wound signal. The antisense plants do produce both PG activity and H2O2 when supplied with systemin, OGA, chitosan, or MJ. A mutant tomato line compromised in the octadecanoid pathway does not exhibit PG activity or H2O2 in response to wounding, systemin, OGA, or chitosan, but does respond to MJ, indicating that the generation of H2O2 requires a functional octadecanoid signaling pathway. Among 18 plant species from six families that were assayed for wound-inducible PG activity and H2O2 generation, 14 species exhibited both wound-inducible PG activity and the generation of H2O2. Four species, all from the Fabaceae family, exhibited little or no wound-inducible PG activity and did not generate H2O2. The time course of wound-inducible PG activity and H2O2 in Arabidopsis thaliana leaves was similar to that found in tomato. The cumulative data suggest that systemic wound signals that induce PG activity and H2O2 are widespread in the plant kingdom and that the response may be associated with the defense of plants against both herbivores and pathogens.
Figures




Similar articles
-
Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate.Plant Cell. 2001 Jan;13(1):179-91. Plant Cell. 2001. PMID: 11158538 Free PMC article.
-
Systemin activates synthesis of wound-inducible tomato leaf polyphenol oxidase via the octadecanoid defense signaling pathway.Proc Natl Acad Sci U S A. 1995 Jan 17;92(2):407-11. doi: 10.1073/pnas.92.2.407. Proc Natl Acad Sci U S A. 1995. PMID: 7831300 Free PMC article.
-
A wound- and systemin-inducible polygalacturonase in tomato leaves.Proc Natl Acad Sci U S A. 1999 Feb 16;96(4):1756-60. doi: 10.1073/pnas.96.4.1756. Proc Natl Acad Sci U S A. 1999. PMID: 9990097 Free PMC article.
-
Polypeptide signaling for plant defensive genes exhibits analogies to defense signaling in animals.Proc Natl Acad Sci U S A. 1996 Oct 29;93(22):12053-8. doi: 10.1073/pnas.93.22.12053. Proc Natl Acad Sci U S A. 1996. PMID: 8901530 Free PMC article. Review.
-
Polypeptide signalling for plant defence genes.Biochem Soc Symp. 1994;60:149-54. Biochem Soc Symp. 1994. PMID: 7639774 Review.
Cited by
-
Plant elicitor peptides are conserved signals regulating direct and indirect antiherbivore defense.Proc Natl Acad Sci U S A. 2013 Apr 2;110(14):5707-12. doi: 10.1073/pnas.1214668110. Epub 2013 Mar 18. Proc Natl Acad Sci U S A. 2013. PMID: 23509266 Free PMC article.
-
Redox Status, JA and ET Signaling Pathway Regulating Responses to Botrytis cinerea Infection Between the Resistant Cucumber Genotype and Its Susceptible Mutant.Front Plant Sci. 2020 Sep 25;11:559070. doi: 10.3389/fpls.2020.559070. eCollection 2020. Front Plant Sci. 2020. PMID: 33101327 Free PMC article.
-
Excess copper induces accumulation of hydrogen peroxide and increases lipid peroxidation and total activity of copper-zinc superoxide dismutase in roots of Elsholtzia haichowensis.Planta. 2008 Jan;227(2):465-75. doi: 10.1007/s00425-007-0632-x. Epub 2007 Oct 2. Planta. 2008. PMID: 17909854
-
A novel superoxide dismutase with a high isoelectric point in higher plants. expression, regulation, and protein localization.Plant Physiol. 2001 Aug;126(4):1668-77. doi: 10.1104/pp.126.4.1668. Plant Physiol. 2001. PMID: 11500564 Free PMC article.
-
Proteinase inhibitor-inducing activity of the prohormone prosystemin resides exclusively in the C-terminal systemin domain.Proc Natl Acad Sci U S A. 1999 Oct 26;96(22):12947-52. doi: 10.1073/pnas.96.22.12947. Proc Natl Acad Sci U S A. 1999. PMID: 10536028 Free PMC article.
References
-
- Doke N, Miura Y, Sanchez L M, Park H-J, Noritake T, Yoshioka H, Kawakita K. Gene. 1996;179:45–51. - PubMed
-
- Lamb C, Dixon R A. Annu Rev Plant Physiol Mol Biol. 1997;48:251–275. - PubMed
-
- Low P S, Merida J R. Plant. 1996;96:533–542.
-
- Alvarez M E, Pennell R I, Meijer P-J, Ishikawa A, Dixon R A, Lamb C. Cell. 1998;92:773–784. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

