Increased vulnerability of postarthritic cartilage to a second arthritic insult: accelerated MMP activity in a flare up of arthritis
- PMID: 10340959
- PMCID: PMC1752891
- DOI: 10.1136/ard.58.6.350
Increased vulnerability of postarthritic cartilage to a second arthritic insult: accelerated MMP activity in a flare up of arthritis
Abstract
Objective: Murine antigen induced arthritis (AIA) is a chronic, smouldering inflammation. Flares of arthritis can be induced by antigen rechallenge or exposure to inflammatory mediators like interleukin 1 (IL1). These flares are characterised by a fast and marked proteoglycan (PG) depletion if compared with the initial arthritis. This study investigated the involvement of metalloproteinases in both the initial and the flare phase of arthritis.
Methods: Murine AIA was induced and a flare up of arthritis was induced by injection of 10 ng of IL1beta. Messenger RNA levels of MMP-1 and -3 were studied by RT-PCR. MMP activity in cartilage, during both primary AIA as well as the flare up of arthritis, was studied by immunodetection of MMP specific neoepitopes in aggrecan (VDIPEN). Cartilage just before flare induction was analysed for presence of MMPs at the mRNA level as well as at the protein level by zymography.
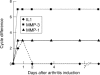
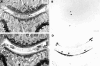
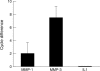
Results: At the onset of AIA, a fast upregulation of mRNA for stromelysin and collagenase was noted. However, no VDIPEN epitopes were detected during this early phase of arthritis. They appeared when PG depletion was severe at day 7 of arthritis and disappeared when cartilage was repaired. IL1 injection into a knee joint at week 4 of AIA caused a flare up of arthritis, coinciding with a fast and marked PG degradation. This degradation was characterised by accelerated expression of VDIPEN epitopes if compared with the expression in primary AIA. Analysis of cartilage at week 4 of AIA showed still increased mRNA levels of MMP-1 and -3. Moreover, increased levels of latent MMPs were present as well, as APMA activation induced profound VDIPEN epitope. In vitro exposure to IL1 did show increased PG breakdown but no VDIPEN expression, suggesting that factors in addition to IL1 are needed to cause the in vivo VDIPEN expression.
Conclusions: The fast and marked PG depletion seen in a flare up of AIA coincides with accelarated expression of MMP induced neoepitopes compared with expression during primary AIA. This accelerated expression is probably linked to increased levels of latent enzyme, which were found to be present in the cartilage before induction of a flare up.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

