Mitogenic signaling by ATP/P2Y purinergic receptors in astrocytes: involvement of a calcium-independent protein kinase C, extracellular signal-regulated protein kinase pathway distinct from the phosphatidylinositol-specific phospholipase C/calcium pathway
- PMID: 10341225
- PMCID: PMC6782585
- DOI: 10.1523/JNEUROSCI.19-11-04211.1999
Mitogenic signaling by ATP/P2Y purinergic receptors in astrocytes: involvement of a calcium-independent protein kinase C, extracellular signal-regulated protein kinase pathway distinct from the phosphatidylinositol-specific phospholipase C/calcium pathway
Abstract
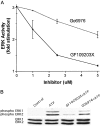
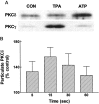
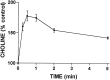
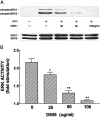
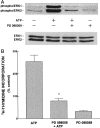
Activation of ATP/P2Y purinergic receptors stimulates proliferation of astrocytes, but the mitogenic signaling pathway linked to these G-protein-coupled receptors is unknown. We have investigated the role of extracellular signal-regulated protein kinase (ERK) in P2Y receptor-stimulated mitogenic signaling as well as the pathway that couples P2Y receptors to ERK. Downregulation of protein kinase C (PKC) in primary cultures of rat cerebral cortical astrocytes greatly reduced the ability of extracellular ATP to stimulate ERK. Because occupancy of P2Y receptors also leads to inositol phosphate formation, calcium mobilization, and PKC activation, we explored the possibility that signaling from P2Y receptors to ERK is mediated by a phosphatidylinositol-specific phospholipase C (PI-PLC)/calcium pathway. However, neither inhibition of PI-PLC nor chelation of calcium significantly reduced ATP-stimulated ERK activity. Moreover, a preferential inhibitor of calcium-dependent PKC isoforms, Gö 6976, was significantly less effective in blocking ATP-stimulated ERK activity than GF102903X, an inhibitor of both calcium-dependent and -independent PKC isoforms. Furthermore, ATP stimulated a rapid translocation of PKCdelta, a calcium-independent PKC isoform, but not PKCgamma, a calcium-dependent PKC isoform. ATP also stimulated a rapid increase in choline, and inhibition of phosphatidylcholine hydrolysis blocked ATP-evoked ERK activation. These results indicate that P2Y receptors in astrocytes are coupled independently to PI-PLC/calcium and ERK pathways and suggest that signaling from P2Y receptors to ERK involves a calcium-independent PKC isoform and hydrolysis of phosphatidylcholine by phospholipase D. In addition, we found that inhibition of ERK activation blocked extracellular ATP-stimulated DNA synthesis, thereby indicating that the ERK pathway mediates mitogenic signaling by P2Y receptors.
Figures

Similar articles
-
Mitogenic signaling from P1 and P2 purinergic receptors to mitogen-activated protein kinase in human fetal astrocyte cultures.Neurosci Lett. 1998 Feb 20;242(3):159-62. doi: 10.1016/s0304-3940(98)00067-6. Neurosci Lett. 1998. PMID: 9530930
-
P2 purinergic receptors signal to glycogen synthase kinase-3beta in astrocytes.J Neurosci Res. 2006 Aug 15;84(3):515-24. doi: 10.1002/jnr.20969. J Neurosci Res. 2006. PMID: 16810687
-
Extracellular ATP activates the PLC/PKC/ERK signaling pathway through the P2Y2 purinergic receptor leading to the induction of early growth response 1 expression and the inhibition of viability in human endometrial stromal cells.Cell Signal. 2008 Jul;20(7):1248-55. doi: 10.1016/j.cellsig.2008.02.011. Epub 2008 Mar 10. Cell Signal. 2008. PMID: 18434089
-
Inositol lipid cycle and autonomous nuclear signalling.Adv Enzyme Regul. 1996;36:101-14. doi: 10.1016/0065-2571(95)00007-0. Adv Enzyme Regul. 1996. PMID: 8869743 Review.
-
Phosphoinositide signaling in unicellular eukaryotes.Crit Rev Microbiol. 2007;33(3):141-56. doi: 10.1080/10408410701415927. Crit Rev Microbiol. 2007. PMID: 17653984 Review.
Cited by
-
Differential frequency dependence of P2Y1- and P2Y2- mediated Ca 2+ signaling in astrocytes.J Neurosci. 2003 Jun 1;23(11):4437-44. doi: 10.1523/JNEUROSCI.23-11-04437.2003. J Neurosci. 2003. PMID: 12805284 Free PMC article.
-
P(2Y) purinoceptor subtypes recruit different mek activators in astrocytes.Br J Pharmacol. 2000 Mar;129(5):927-36. doi: 10.1038/sj.bjp.0703138. Br J Pharmacol. 2000. PMID: 10696092 Free PMC article.
-
Electroacupuncture Inhibits the Activity of Astrocytes in Spinal Cord in Rats with Visceral Hypersensitivity by Inhibiting P2Y1 Receptor-Mediated MAPK/ERK Signaling Pathway.Evid Based Complement Alternat Med. 2020 Feb 25;2020:4956179. doi: 10.1155/2020/4956179. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32184891 Free PMC article.
-
Regulation of phospholipase D activity and phosphatidic acid production after purinergic (P2Y6) receptor stimulation.J Biol Chem. 2013 Jul 12;288(28):20477-87. doi: 10.1074/jbc.M113.451708. Epub 2013 May 30. J Biol Chem. 2013. PMID: 23723068 Free PMC article.
-
Purinergic signaling induces thrombospondin-1 expression in astrocytes.Proc Natl Acad Sci U S A. 2006 Jun 13;103(24):9321-6. doi: 10.1073/pnas.0603146103. Epub 2006 Jun 5. Proc Natl Acad Sci U S A. 2006. PMID: 16754856 Free PMC article.
References
-
- Abbracchio MP, Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther. 1994;64:445–475. - PubMed
-
- Abbracchio MP, Saffrey MJ, Hopker V, Burnstock G. Modulation of astroglial cell proliferation by analogues of adenosine and ATP in primary cultures of rat striatum. Neuroscience. 1994;59:67–76. - PubMed
-
- Abbracchio MP, Ceruti S, Langfelder R, Cattabeni F, Saffrey MJ, Burnstock G. Effects of ATP analogues and basic fibroblast growth factor on astroglial cell differentiation in primary cultures of rat striatum. Int J Dev Neurosci. 1995;13:685–693. - PubMed
-
- Ahn NG, Weie JE, Chan CP, Krebs EG. Identification of multiple epidermal growth factor-stimulated protein serine/threonine kinases from Swiss 3T3 cells. J Biol Chem. 1990;265:11487–11494. - PubMed
-
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
