Sensory impairments and delayed regeneration of sensory axons in interleukin-6-deficient mice
- PMID: 10341234
- PMCID: PMC6782624
- DOI: 10.1523/JNEUROSCI.19-11-04305.1999
Sensory impairments and delayed regeneration of sensory axons in interleukin-6-deficient mice
Abstract
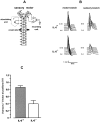
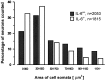
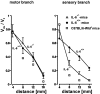
Interleukin-6 (IL-6) is a multifunctional cytokine mediating inflammatory or immune reactions. Here we investigated the possible role of IL-6 in the intact or lesioned peripheral nervous system using adult IL-6 gene knockout (IL-6(-/-)) mice. Various sensory functions were tested by applying electrophysiological, morphological, biochemical, and behavioral methods. There was a 60% reduction of the compound action potential of the sensory branch of IL-6(-/-) mice as compared with the motor branch in the intact sciatic nerve. Cross sections of L5 DRG of IL-6(-/-) mice showed a shift in the relative size distribution of the neurons. The temperature sensitivity of IL-6(-/-) mice was also significantly reduced. After crush lesion of the sciatic nerve, its functional recovery was delayed in IL-6(-/-) mice as analyzed from a behavioral footprint assay. Measurements of compound action potentials 20 d after crush lesion showed that there was a very low level of recovery of the sensory but not of the motor branch of IL-6(-/-) mice. Similar results of sensory impairments were obtained with mice showing slow Wallerian degeneration (Wlds) and a delayed lesion-induced recruitment of macrophages. However, in contrast to WldS mice, in IL-6(-/-) mice we observed the characteristic lesion-induced invasion of macrophages and the upregulation of low-affinity neurotrophin receptor p75 (p75LNTR) mRNA levels identical to those of IL-6(+/+) mice. Thus, the mechanisms leading to the common sensory deficiencies were different between IL-6(-/-) and WldS mice. Altogether, the results suggest that interleukin-6 is essential to modulate sensory functions in vivo.
Figures





Similar articles
-
Delayed wallerian degeneration in sciatic nerves of C57BL/Ola mice is associated with impaired regeneration of sensory axons.Brain Res. 1990 Oct 15;530(1):117-20. doi: 10.1016/0006-8993(90)90666-y. Brain Res. 1990. PMID: 2271939
-
Further studies on motor and sensory nerve regeneration in mice with delayed Wallerian degeneration.Eur J Neurosci. 1994 Mar 1;6(3):420-8. doi: 10.1111/j.1460-9568.1994.tb00285.x. Eur J Neurosci. 1994. PMID: 8019679
-
Conditioning injury-induced spinal axon regeneration fails in interleukin-6 knock-out mice.J Neurosci. 2004 May 5;24(18):4432-43. doi: 10.1523/JNEUROSCI.2245-02.2004. J Neurosci. 2004. PMID: 15128857 Free PMC article.
-
Consequences of slow Wallerian degeneration for regenerating motor and sensory axons.J Neurobiol. 1992 Jul;23(5):521-36. doi: 10.1002/neu.480230507. J Neurobiol. 1992. PMID: 1431835
-
[Sensitivity: physiology, examination, principles of rehabilitation of sensation].Ann Chir Main. 1987;6(3):230-8. doi: 10.1016/s0753-9053(87)80065-0. Ann Chir Main. 1987. PMID: 3322218 Review. French.
Cited by
-
Interleukin-6 Deficiency Attenuates Retinal Ganglion Cell Axonopathy and Glaucoma-Related Vision Loss.Front Neurosci. 2017 May 31;11:318. doi: 10.3389/fnins.2017.00318. eCollection 2017. Front Neurosci. 2017. PMID: 28620279 Free PMC article.
-
The cytokine interleukin-6 is sufficient but not necessary to mimic the peripheral conditioning lesion effect on axonal growth.J Neurosci. 2006 May 17;26(20):5565-73. doi: 10.1523/JNEUROSCI.0815-06.2006. J Neurosci. 2006. PMID: 16707807 Free PMC article.
-
The role of interleukin-1, interleukin-6, and glia in inducing growth of neuronal terminal arbors in mice.J Neurosci. 2002 Sep 15;22(18):8034-41. doi: 10.1523/JNEUROSCI.22-18-08034.2002. J Neurosci. 2002. PMID: 12223557 Free PMC article.
-
The nuclear receptor ROR(alpha) exerts a bi-directional regulation of IL-6 in resting and reactive astrocytes.Proc Natl Acad Sci U S A. 2009 Dec 15;106(50):21365-70. doi: 10.1073/pnas.0911782106. Epub 2009 Dec 1. Proc Natl Acad Sci U S A. 2009. PMID: 19955433 Free PMC article.
-
Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation.J Neurosci. 2005 Feb 16;25(7):1645-53. doi: 10.1523/JNEUROSCI.3269-04.2005. J Neurosci. 2005. PMID: 15716400 Free PMC article.
References
-
- Akira S, Kishimoto T. Role of interleukin-6 in macrophage function. Curr Opin Hematol. 1996;3:87–93. - PubMed
-
- Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL-6 and related molecules (IL 1 and TNF). FASEB J. 1990;4:2860–2867. - PubMed
-
- Austyn JM, Gordon S. F4/80: a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;10:805–815. - PubMed
-
- Barnum SR, Jones JL, Müller-Ladner U, Samimi A, Campbell IL. Chronic complement C3 gene expression in the CNS of transgenic mice with astrocyte-targeted interleukin-6 expression. Glia. 1996;18:107–117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
